
Synopsis
0
CEP/COS
0
VMF
0
Weekly News Recap #Phispers
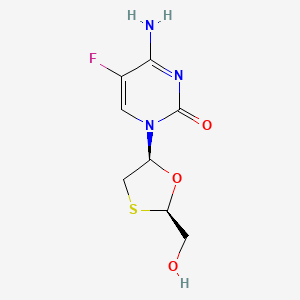

1. Beta L 2',3' Dideoxy 5 Fluoro 3' Thiacytidine
2. Beta-l-2',3'-dideoxy-5-fluoro-3'-thiacytidine
3. Coviracil
4. Emtriva
1. 143491-57-0
2. Emtriva
3. Coviracil
4. (-)-ftc
5. 4-amino-5-fluoro-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1h)-one
6. 143491-54-7
7. 524w91
8. Bw-524w91
9. Ftc
10. Racivir
11. 4-amino-5-fluoro-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one
12. Bw524w91
13. (-)-2'-deoxy-5-fluoro-3'-thiacytidine
14. Ftc-(-)
15. Bw 524w91
16. (-)-emtricitabine
17. (2r-cis)-4-amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1h)-pyrimidinone
18. (-)-beta-2',3'-dideoxy-5-fluoro-3'-thiacytidine
19. Chebi:31536
20. 5-fluoro-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine
21. Emtricitabine, (-)-
22. Psi-5004
23. 4-amino-5-fluoro-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1h)-one
24. 145213-48-5
25. G70b4etf4s
26. Uls8902u4o
27. Emtritabine
28. Bw1592
29. (-)-cis-4-amino-5-fluoro-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1h)-pyrimidin-2-one
30. 2',3'-dideoxy-5-fluoro-3'-thiacytidine
31. Ncgc00164564-01
32. (-)-(2r,5s)-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine
33. 2(1h)-pyrimidinone, 4-amino-5-fluoro-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, Rel-
34. 524-w-91
35. Psi 5004
36. Mfcd00870151
37. Ftc, Dl-
38. Coviracil(tm)
39. Drg-0208
40. 4-amino-5-fluoro-1-((2r,5s)-2-hydroxymethyl-[1,3]oxathiolan-5-yl)-1h-pyrimidin-2-one
41. Rel-4-amino-5-fluoro-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1h)-pyrimidinone
42. Emtriva(tm)
43. 2(1h)-pyrimidinone, 4-amino-5-fluoro-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-, Rel-
44. Emtricitabine (emtriva)
45. Smr002533604
46. Bw 1592
47. 2',3',5-ftc
48. Emtricitabine, (+/-)-
49. Hsdb 7337
50. 4-amino-5-fluoro-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-1,2-dihydropyrimidin-2-one
51. Ftc, (-)-
52. Unii-g70b4etf4s
53. Emtricitabine [usan:inn]
54. (-)-.beta.-l-ftc
55. Unii-uls8902u4o
56. Emtricitabinum
57. (+/-)-ftc
58. 2(1h)-pyrimidinone,4-amino-5-fluoro-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-
59. 2(1h)-pyrimidinone,4-amino-5-fluoro-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-
60. Emtricitabine- Bio-x
61. Emtricitabine [mi]
62. Chembl885
63. Emtricitabine [inn]
64. Emtricitabine [jan]
65. 2(1h)-pyrimidinone, 4-amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, Cis-
66. Dsstox_cid_20129
67. Dsstox_rid_79445
68. Emtricitabine [hsdb]
69. Emtricitabine [usan]
70. Dsstox_gsid_40129
71. Schembl39708
72. Emtricitabine [vandf]
73. Mls003882429
74. Mls006011556
75. Mls006011987
76. Emtricitabine [mart.]
77. Emtricitabine [usp-rs]
78. Emtricitabine [who-dd]
79. Emtricitabine [who-ip]
80. Dtxsid0040129
81. Emtricitabine [ema Epar]
82. Gtpl11244
83. Ex-a150
84. Hms2089i05
85. Hms3713l12
86. Emtricitabine [orange Book]
87. Zinc3629271
88. Tox21_112193
89. ((-))-ftc
90. Bdbm50107843
91. Cs1125
92. Ftc-((-))
93. Nsc804863
94. S1704
95. Atripla Component Emtricitabine
96. Descovy Component Emtricitabine
97. Emtricitabinum [who-ip Latin]
98. Odefsey Component Emtricitabine
99. Truvada Component Emtricitabine
100. Akos015853098
101. Akos015894950
102. Eviplera Component Emtricitabine
103. Stribild Component Emtricitabine
104. Am84393
105. Ccg-220615
106. Cs-1370
107. Db00879
108. Nsc-804863
109. 2',3'-dideoxy-3-thia-5-fluorocytidine
110. Descovy Component Of Emtricitabine
111. Emtricitabine Component Of Atripla
112. Emtricitabine Component Of Descovy
113. Emtricitabine Component Of Genvoya
114. Emtricitabine Component Of Odefsey
115. Emtricitabine Component Of Truvada
116. Ncgc00164564-06
117. Ncgc00164564-09
118. Ncgc00164564-10
119. 2(1h)-pyrimidinone, 4-amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, (2r-cis)-
120. 2(1h)-pyrimidinone, 4-amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, Cis-(+-)-
121. As-14099
122. Be165946
123. Emtricitabine Component Of Eviplera
124. Emtricitabine Component Of Stribild
125. Hy-17427
126. Cas-143491-57-0
127. E1007
128. Emtricitabine 100 Microg/ml In Acetonitrile
129. Sw220172-1
130. D01199
131. D72669
132. Ent-emtricitabine; Emtricitabine Ip Impurity D
133. Ab01275429-01
134. 491e570
135. Q422604
136. W-201247
137. W-201248
138. 2',3'-dideoxy-5-fluoro-3'-thiacytidine, Dl-
139. Z1739256297
140. .beta.-l-(-)-(2r,5s)-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine
141. 5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine (2r,5s)
142. 2(1h)-pyrimidinone, 4-amino-5-fluoro-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-
143. 4-amino-5-fluoro-1-[(2s,5r)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1h)-pyrimidinone; (+)-2'-deoxy-3'-thia-5-fluorocytidine; (2s-cis)-4-amino-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1h)-pyrimidinone
144. 5-fluoro-(-)-l-2',3'-dideoxy-3'-thiacytidine; (-)-beta-2',3'-dideoxy-5-fluoro-3'-thiacytidine; Emtricitabine; Emtriva
| Molecular Weight | 247.25 g/mol |
|---|---|
| Molecular Formula | C8H10FN3O3S |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 247.04269053 g/mol |
| Monoisotopic Mass | 247.04269053 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 374 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Emtricitabine |
| PubMed Health | Emtricitabine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | EMTRIVA is the brand name of emtricitabine, a synthetic nucleoside analog with activity against human immunodeficiency virus type 1 (HIV-1) reverse transcriptase.The chemical name of emtricitabine is 5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiol... |
| Active Ingredient | Emtricitabine |
| Dosage Form | Capsule |
| Route | oral |
| Strength | 200mg |
| Market Status | Tentative Approval |
| Company | Matrix Labs; Aurobindo Pharma; Cipla |
| 2 of 4 | |
|---|---|
| Drug Name | Emtriva |
| PubMed Health | Emtricitabine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | EMTRIVA is the brand name of emtricitabine, a synthetic nucleoside analog with activity against human immunodeficiency virus type 1 (HIV-1) reverse transcriptase.The chemical name of emtricitabine is 5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiol... |
| Active Ingredient | Emtricitabine |
| Dosage Form | Capsule; Solution |
| Route | oral; Oral |
| Strength | 200mg; 10mg/ml |
| Market Status | Prescription |
| Company | Gilead |
| 3 of 4 | |
|---|---|
| Drug Name | Emtricitabine |
| PubMed Health | Emtricitabine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | EMTRIVA is the brand name of emtricitabine, a synthetic nucleoside analog with activity against human immunodeficiency virus type 1 (HIV-1) reverse transcriptase.The chemical name of emtricitabine is 5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiol... |
| Active Ingredient | Emtricitabine |
| Dosage Form | Capsule |
| Route | oral |
| Strength | 200mg |
| Market Status | Tentative Approval |
| Company | Matrix Labs; Aurobindo Pharma; Cipla |
| 4 of 4 | |
|---|---|
| Drug Name | Emtriva |
| PubMed Health | Emtricitabine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | EMTRIVA is the brand name of emtricitabine, a synthetic nucleoside analog with activity against human immunodeficiency virus type 1 (HIV-1) reverse transcriptase.The chemical name of emtricitabine is 5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiol... |
| Active Ingredient | Emtricitabine |
| Dosage Form | Capsule; Solution |
| Route | oral; Oral |
| Strength | 200mg; 10mg/ml |
| Market Status | Prescription |
| Company | Gilead |
Antiviral Agents
National Library of Medicine's Medical Subject Headings. Emtricitabine. Online file (MeSH, 2014). Available from, as of November 19, 2013: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Emtricitabine is used in conjunction with other antiretroviral agents for the treatment of HIV-1 infection in pediatric patients 12 years of age or older. The fixed combination containing emtricitabine and tenofovir (Truvada) can be used in conjunction with other antiretrovirals in children 12 years of age or older weighing at least 35 kg. For initial treatment of HIV-infected pediatric patients, the HHS Panel on Antiretroviral Therapy and Medical Management of HIV-infected Children recommends antiretroviral therapy with at least 3 drugs, including either a PI or NNRTI with 2 NRTIs. When PI-based or NNRTI-based regimens are used in pediatric patients, emtricitabine and (abacavir (for individuals 3 months of age or older who test negative for the HLA-B*5701 allele), tenofovir (for adolescents 12 years of age or older at Tanner stage 4 or 5), or zidovudine) are preferred dual NRTI options. A dual NRTI combination of emtricitabine and lamivudine is not recommended, because of similar resistance profiles and minimal additive antiretroviral activity. A triple NRTI regimen that includes abacavir, tenofovir, and either lamivudine or emtricitabine or a triple NRTI regimen that includes tenofovir, didanosine, and either lamivudine or emtricitabine should not be used at any time in pediatric patients because of the high rate of early virologic failure reported in antiretroviral-naive adults.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 700
Emtricitabine has been evaluated in a randomized, open-label, multicenter study (study 303) in 440 previously treated adults (mean age: 42 years; 86% male; 64% white; 13% Hispanic; 21% African American; median baseline plasma HIV-1 RNA level: 1.7 log10 copies/mL; mean baseline CD4+ T-cell count: 527 cells/mm3) who had received a lamivudine-containing regimen that also included 2 other antiretrovirals (background regimen) for at least 12 weeks prior to study entry and had plasma HIV-1 levels of 400 copies/mL or less. Patients in this study were randomized to receive emtricitabine in conjunction with stavudine or zidovudine and PI or NNRTI or to continue their lamivudine-containing background regimen (i.e., lamivudine in conjunction with stavudine or zidovudine and a PI or NNRTI). At week 48, 77 and 67% of adults receiving the regimen that included emtricitabine and 82 and 72% of those receiving the regimen that included lamivudine had plasma HIV-1 RNA levels less than 400 or 50 copies/mL, respectively. Virologic failure (i.e., individuals who failed to achieve virologic suppression or experienced rebound after achieving virologic suppression) was reported in 7% of those receiving the emtricitabine-containing regimen and in 8% of those receiving the lamivudine-containing regimen at week 48. Administration of the emtricitabine-containing regimen resulted in a mean increase in CD4+ T-cell counts of 29 cells/mm3; administration of the lamivudine-containing regimen resulted in a mean increase in CD4+ T-cell counts of 61 cells/cu mm.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 700
Safety and efficacy of emtricitabine used in conjunction with other antiretrovirals have been evaluated in a randomized multicenter study in treatment-naive adults (study 301A). In this study, 571 HIV-infected adults (mean age: 36 years; 85% male; 52% white; 26% Hispanic; 16% African American; median baseline plasma HIV-1 RNA level: 4.9 log10 copies/mL; mean baseline CD4+ T-cell count: 318 cells/mm3) were randomized to receive emtricitabine or stavudine in conjunction with didanosine (delayed-release capsules) and efavirenz. Results of this study indicated that an initial regimen that includes emtricitabine in conjunction with didanosine and efavirenz is as effective as an initial regimen of stavudine in conjunction with didanosine and efavirenz. At week 48, 81 and 78% of adults receiving the regimen that included emtricitabine and 68 and 59% of those receiving the regimen that included stavudine had plasma HIV-1 RNA levels less than 400 or 50 copies/mL, respectively. Virologic failure (i.e., individuals who failed to achieve virologic suppression or experienced rebound after achieving virologic suppression) at week 48 was reported in 3% of those receiving the emtricitabine-containing regimen and in 11% of those receiving the stavudine-containing regimen. At week 48, increases in CD4+ T-cell counts were greater in patients receiving the regimen that included emtricitabine (mean increase of 168 cells/mm3) than in those receiving the regimen that included stavudine (mean increase of 134 cells/mm3).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 700
For more Therapeutic Uses (Complete) data for EMTRICITABINE (7 total), please visit the HSDB record page.
Safety and efficacy of emtricitabine have been established for treatment of HIV-1 infection in children 3 months of age and older. The pharmacokinetics and safety of emtricitabine were evaluated in a dose-finding study in 20 neonates born to HIV-infected mothers.20 1 These neonates received zidovudine prophylaxis for 6 weeks. In addition, these neonates received 2 short courses of emtricitabine (3 mg/kg daily for 4 days per course) during the first 12 weeks of life. This dose was well tolerated and was not associated with any safety issues. Systemic exposure (area under the plasma concentration-time curve [AUC]) in infants 0-3 months of age receiving emtricitabine 3 mg/kg daily was similar to that reported in children 3 months to 17 years of age receiving emtricitabine 6 mg/kg daily. All neonates were HIV-1 negative at the end of the study (6 months postpartum); efficacy of emtricitabine for the prevention or treatment of HIV was not determined.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 703
Lactic acidosis and severe hepatomegaly with steatosis, including fatalities, have been reported in patients receiving nucleoside reverse transcriptase inhibitors (NRTIs), including emtricitabine, in conjunction with other antiretroviral agents. Most reported cases have involved women; obesity and long-term therapy with a nucleoside analog also may be risk factors.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 702
Caution should be observed when nucleoside reverse transcriptase inhibitors are used in patients with known risk factors for liver disease; however, lactic acidosis and severe hepatomegaly with steatosis have been reported in patients with no known risk factors. Emtricitabine therapy should be interrupted in any patient with clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (signs of hepatotoxicity include hepatomegaly and steatosis even in the absence of marked increases in serum aminotransferase concentrations).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 702
Prior to initiation of emtricitabine therapy for the treatment of HIV-1 infection, patients should be tested for chronic hepatitis B virus (HBV). Emtricitabine is not indicated for the treatment of chronic HBV infection; safety and efficacy of the drug have not been established in patients with coexisting HBV and HIV infections. Exacerbations of HBV infection have been reported in HIV-infected patients following discontinuance of emtricitabine. Patients with HBV infection and HIV infection should be closely monitored with clinical and laboratory follow-up for at least several months after stopping emtricitabine therapy.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 702
For more Drug Warnings (Complete) data for EMTRICITABINE (13 total), please visit the HSDB record page.
Emtricitabine is indicated in combination with other medications for the treatment of HIV-1 infections; treatment of HIV-1 infections in pediatric patients 25-35kg, treatment of HIV-1 infections in adult patients 35kg, for pre exposure prophylaxis of HIV-1 in adolescent and adult patients excluding those who have receptive vaginal sex; treatment of HIV-1 infections in pediatric and adult patients 17kg, pre exposure prophylaxis in adolescents and adults 35kg; treatment of HIV-1 in patients 12 years and 35kg; treatment of HIV-1 in patients weighing 35kg; treatment of HIV-1 in patients weighing 25kg; and treatment of HIV-1 in patients weighing 40kg.
FDA Label
Emtriva is indicated for the treatment of HIV-1 infected adults and children in combination with other antiretroviral agents.
This indication is based on studies in treatment-naive patients and treatment-experienced patients with stable virological control. There is no experience of the use of Emtriva in patients who are failing their current regimen or who have failed multiple regimens.
When deciding on a new regimen for patients who have failed an antiretroviral regimen, careful consideration should be given to the patterns of mutations associated with different medicinal products and the treatment history of the individual patient. Where available, resistance testing may be appropriate.
Emtricitabine is a cytidine analog that competes with the natural substrate of HIV-1 reverse transcriptase to be incorporated into newly formed DNA, terminating its transcription. It is administered once daily so it has a long duration of action. Patients should be counselled regarding the risk of lactic acidosis and hepatomegaly with steatosis.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J05AF09
J05AF09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
J05AF09 - Emtricitabine
Absorption
Emtricitabine reaches a Cmax of 1.80.7g/mL with a Tmax of 1-2 hours, and has an AUC of 103.1g\*hr/mL. The bioavailability of emtricitabine capsules is 93% and the bioavailability of the oral solution is 75%. Taking emtricitabine with food decreases the Cmax by 29%.[L9019
Route of Elimination
Emtricitabine is 86% recovered in the urine and 14% recovered in feces. 13% of the dose is recovered in the urine as metabolites; 9% as 3'-sulfoxide diastereomers and 4% as 2'-O-glucuronide.
Volume of Distribution
The apparent central volume of distribution is 42.3L and the peripheral volume of distribution is 55.4L.
Clearance
Emtricitabine has an apparent elimination rate of 15.1L/h. This rate is closely linked to creatinine clearance.
Emtricitabine is rapidly and extensively absorbed following oral administration with peak plasma concentrations occurring at 1 to 2 hours post-dose. Following multiple dose oral administration of emtriva to 20 HIV-infected subjects, the (mean + or - SD) steady state plasma emtricitabine peak concentration (Cmax) was 1.8 + or 0.7 ug/mL and the area under the plasma concentration-time curve over a 24-hour dosing interval (AUC) was 10.0 + or - 3.1 hr/ug/mL. The mean steady state plasma trough concentration at 24 hours post-dose was 0.09 ug/mL. The mean absolute bioavailability of emtriva was 93%.
Physicians Desk Reference 65th ed. PDR Network, LLC, Montvale, NJ. 2011, p. 1154
In vitro binding of emtricitabine to human plasma proteins was <4% and independent of concentration over the range of 0.02 to 200 ug/mL. At peak plasma concentration, the mean plasma to blood drug concentration ratio was approximately 1.0 and the mean semen to plasma drug concentration ratio was approximately 4.0.
Physicians Desk Reference 65th ed. PDR Network, LLC, Montvale, NJ. 2011, p. 1155
Time to peak concentration: 1 to 2 hours post dose
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1265
Emtricitabine is distributed into human milk in low concentrations.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 703
For more Absorption, Distribution and Excretion (Complete) data for EMTRICITABINE (6 total), please visit the HSDB record page.
Emtricitabine is approximately 86% unmetabolized. Approximately 9% of a dose is metabolized to 3'-sulfoxide diastereomers, 4% to the 2'-O-glucuronide, and a minor amount is converted to 5-fluorocytosine.
The biotransformation of emtricitabine includes oxidation of the thiol moiety to form the 3'-sulfoxide diastereomers (approximately 9% of dose) and conjugation with glucuronic acid to form 2'-O-glucuronide (approximately 4% of dose). No other metabolites were identifiable. Emtricitabine is not metabolized by liver enzymes.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1265
The half life of emtricitabine is approximately 10 hours.
The plasma emtricitabine half-life is approximately 10 hours.
Physicians Desk Reference 65th ed. PDR Network, LLC, Montvale, NJ. 2011, p. 1155
Emtricitabine is a cytidine analog which, when phosphorylated to emtricitabine 5'-triphosphate, competes with deoxycytidine 5'-triphosphate for HIV-1 reverse transcriptase. As HIV-1 reverse transcriptase incorporates emtricitabine into forming DNA strands, new nucleotides are unable to be incorporated, leading to viral DNA chain termination. Inhibition of reverse transcriptase prevents transcription of viral RNA into DNA, therefore the virus is unable to incorporate its DNA into host DNA and replicate using host cell machinery. This reduces viral load.
Emtricitabine, a synthetic nucleoside analog of cytosine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 reverse transcriptase by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA which results in chain termination. Emtricitabine 5'-triphosphate is a weak inhibitor of mammalian DNA polymerase alpha, beta, epsilon and mitochondrial DNA polymerase gamma.
Physicians Desk Reference 65th ed. PDR Network, LLC, Montvale, NJ. 2011, p. 1155
Date of Issue : 2023-12-26
Valid Till : 2026-12-03
Written Confirmation Number : WC-0487
Address of the Firm : Plot No. 48-50, 209-211, IDA, Phase-ll, Pashamylaram, Patancheru, Sangareddy Dis...

Date of Issue : 2025-07-11
Valid Till : 2028-06-25
Written Confirmation Number : WC-0023
Address of the Firm : Sy. No\'s. 52,53,58,59,61 to 78, 127 & 128, Pydibhimavaram Village & Sy. No\'s. ...

Date of Issue : 2025-06-19
Valid Till : 2028-07-02
Written Confirmation Number : WC-0141
Address of the Firm : Plot No. D-27, M.I.D.C, Industrial Area, Kurkumbh, Taluka

Date of Issue : 2024-02-19
Valid Till : 2027-02-18
Written Confirmation Number : WC-0578
Address of the Firm : Sy.No.405 & 408, Veliminedu (Village), Chityal (Mandal), Nalgonda District - 508...

Date of Issue : 2025-09-03
Valid Till : 2028-08-08
Written Confirmation Number : WC-0041
Address of the Firm : Unit-I, Sy. No. 10, I.D.A, Gaddapotharam (V), Jinnaram (M), Sangareddy District,...

Date of Issue : 2022-09-30
Valid Till : 2025-08-15
Written Confirmation Number : WC-0065
Address of the Firm : Unit-IX, Plot No. 2, Hetero Infrastructure- SEZ Ltd., N. Narasapuram (Village), ...

Date of Issue : 2024-08-04
Valid Till : 2026-03-22
Written Confirmation Number : WC-0395
Address of the Firm : Plot No. 18, Jawaharlal Nehru Pharma City, Parawada Mandal, Anakapalli District ...

Date of Issue : 2022-09-30
Valid Till : 2025-09-10
Written Confirmation Number : WC-0201
Address of the Firm : T-142, MIDC, Tarapur, Boisar, Palghar - 401506, Maharashtra, India

Date of Issue : 2025-11-12
Valid Till : 2028-11-10
Written Confirmation Number : WC-0079
Address of the Firm : Plot No. - 2209, G.I.D.C., Sarigam - 396 155, Dist - Valsad, Gujarat, India

Emtricitabine IH/IP/USP/Ph. Int
Date of Issue : 2024-03-21
Valid Till : 2027-03-20
Written Confirmation Number : WC-0473
Address of the Firm : Unit-2, Plot No 1203, Ill Phase GIDC, Vapi -396195, Dist.-Valsad, Gujarat, India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
RLD : No
TE Code : AB
EMTRICITABINE; TENOFOVIR DISOPROXIL FUMARATE
Brand Name : EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG;300MG
Approval Date : 2021-01-13
Application Number : 91055
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
EMTRICITABINE; TENOFOVIR DISOPROXIL FUMARATE
Brand Name : EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG;150MG
Approval Date : 2018-08-22
Application Number : 209721
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
Brand Name : EMTRICITABINE AND TENOFOVIR ALAFENAMIDE FUMARATE
Dosage Form : TABLET;ORAL
Dosage Strength : 120MG;EQ 15MG BASE
Approval Date : 2024-05-17
Application Number : 214053
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
EMTRICITABINE; TENOFOVIR DISOPROXIL FUMARATE
Brand Name : EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG;300MG
Approval Date : 2018-01-26
Application Number : 90513
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
EMTRICITABINE, TENOFOVIR ALAFENAMIDE
Brand Name : EMTRICITABINE AND TENOFOVIR ALAFENAMIDE
Dosage Form : TABLET
Dosage Strength : 15MG
Approval Date :
Application Number : 211680
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
EMTRICITABINE; TENOFOVIR DISOPROXIL FUMARATE
Brand Name : TRUVADA
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG;150MG
Approval Date : 2016-03-10
Application Number : 21752
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
EMTRICITABINE; RILPIVIRINE HYDROCHLORIDE; TENOFOVIR DISOPROXIL FUMARATE
Brand Name : COMPLERA
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG;EQ 25MG BASE;300MG
Approval Date : 2011-08-10
Application Number : 202123
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
Brand Name : EMTRICITABINE AND TENOFOVIR ALAFENAMIDE FUMARATE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG;EQ 25MG BASE
Approval Date : 2024-12-13
Application Number : 213926
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : EMTRICITABINE
Dosage Form : CAPSULE; ORAL
Dosage Strength : 200MG
Approval Date :
Application Number : 90488
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
EMTRICITABINE; TENOFOVIR DISOPROXIL FUMARATE; NEVIRAPINE
Brand Name : EMTRICITABINE;TENOFOVIR DISOPROXIL FUMARATE;NEVIRAPINE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG;300MG;200MG
Approval Date :
Application Number : 206042
RX/OTC/DISCN :
RLD :
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Truvada
Dosage Form : TAB
Dosage Strength : 200mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Tencitab
Dosage Form : TAB
Dosage Strength : 200mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Tribuss
Dosage Form : FCT
Dosage Strength : 200mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Teerenz
Dosage Form : TAB
Dosage Strength : 200mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Adco Emtevir
Dosage Form : TAB
Dosage Strength : 200mg
Packaging : 60X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Rizene
Dosage Form : FCT
Dosage Strength : 300mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Altaeda
Dosage Form : FCT
Dosage Strength : 200mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : TABEX
Dosage Form : FCT
Dosage Strength : 200mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : HETRIPCO
Dosage Form : FCT
Dosage Strength : 200mg
Packaging : 90X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Triemta Tablets
Dosage Form : FCT
Dosage Strength : 200mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Alafenamide
Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Alafenamide
Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 196
2019 Revenue in Millions : 369
Growth (%) : -47

Bictegravir, Emtricitabine, Tenofovir Alafenamide
Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 3,338
2019 Revenue in Millions : 3,931
Growth (%) : -15

Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Alafenamide
Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Alafenamide
Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 2,184
2019 Revenue in Millions : 2,110
Growth (%) : 4

Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 349
2019 Revenue in Millions : 600
Growth (%) : -42

Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 269
2019 Revenue in Millions : 406
Growth (%) : -34

Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Alafenamide
Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Alafenamide
Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 3,338
2019 Revenue in Millions : 3,931
Growth (%) : -15

Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 3,338
2019 Revenue in Millions : 3,931
Growth (%) : -15

Bictegravir, Emtricitabine, Tenofovir Alafenamide
Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 488
2019 Revenue in Millions : 379
Growth (%) : 29

Bictegravir, Emtricitabine, Tenofovir Alafenamide
Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 1,861
2019 Revenue in Millions : 1,500
Growth (%) : 24

Main Therapeutic Indication : Infectious Diseases
Currency : USD
2020 Revenue in Millions : 488
2019 Revenue in Millions : 379
Growth (%) : 29

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
20 Jun 2023
Reply
25 Jul 2022
Reply
14 Jul 2022
Reply
29 Jun 2022
Reply
25 Nov 2019
Reply
13 Jul 2019
Reply
21 Oct 2018
Reply
31 Jul 2018
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
Patent Expiration Date : 2033-12-19
BICTEGRAVIR SODIUM; EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
US Patent Number : 9216996
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 210251
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2033-12-19

Patent Expiration Date : 2025-12-09
EMTRICITABINE; RILPIVIRINE HYDROCHLORIDE; TENOFOVIR DISOPROXIL FUMARATE
US Patent Number : 8841310
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 202123
Patent Use Code : U-257
Delist Requested :
Patent Use Description : TREATMENT OF HIV INFEC...
Patent Expiration Date : 2025-12-09

Patent Expiration Date : 2025-07-07
EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
US Patent Number : 7390791*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 208215
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2025-07-07

Patent Expiration Date : 2027-02-27
COBICISTAT; ELVITEGRAVIR; EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
US Patent Number : 7176220*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 207561
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2027-02-27

Patent Expiration Date : 2025-07-07
BICTEGRAVIR SODIUM; EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
US Patent Number : 7390791*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 210251
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2025-07-07

Patent Expiration Date : 2030-10-30
COBICISTAT; ELVITEGRAVIR; EMTRICITABINE; TENOFOVIR DISOPROXIL FUMARATE
US Patent Number : 8633219*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 203100
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-10-30

Patent Expiration Date : 2025-04-21
EMTRICITABINE; RILPIVIRINE HYDROCHLORIDE; TENOFOVIR DISOPROXIL FUMARATE
US Patent Number : 7125879
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 202123
Patent Use Code : U-257
Delist Requested :
Patent Use Description : TREATMENT OF HIV INFEC...
Patent Expiration Date : 2025-04-21

Patent Expiration Date : 2032-08-15
EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
US Patent Number : 9296769
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 208215
Patent Use Code : U-1663
Delist Requested :
Patent Use Description : TREATMENT OF HIV-1 INF...
Patent Expiration Date : 2032-08-15

Patent Expiration Date : 2033-12-19
BICTEGRAVIR SODIUM; EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
US Patent Number : 9216996
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 210251
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2033-12-19

Patent Expiration Date : 2030-04-30
COBICISTAT; ELVITEGRAVIR; EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE
US Patent Number : 8633219
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 207561
Patent Use Code : U-257
Delist Requested :
Patent Use Description : TREATMENT OF HIV INFEC...
Patent Expiration Date : 2030-04-30

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
100
PharmaCompass offers a list of Emtricitabine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Emtricitabine manufacturer or Emtricitabine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Emtricitabine manufacturer or Emtricitabine supplier.
PharmaCompass also assists you with knowing the Emtricitabine API Price utilized in the formulation of products. Emtricitabine API Price is not always fixed or binding as the Emtricitabine Price is obtained through a variety of data sources. The Emtricitabine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Emtricitabine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Emtricitabine, including repackagers and relabelers. The FDA regulates Emtricitabine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Emtricitabine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Emtricitabine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Emtricitabine supplier is an individual or a company that provides Emtricitabine active pharmaceutical ingredient (API) or Emtricitabine finished formulations upon request. The Emtricitabine suppliers may include Emtricitabine API manufacturers, exporters, distributors and traders.
click here to find a list of Emtricitabine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Emtricitabine DMF (Drug Master File) is a document detailing the whole manufacturing process of Emtricitabine active pharmaceutical ingredient (API) in detail. Different forms of Emtricitabine DMFs exist exist since differing nations have different regulations, such as Emtricitabine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Emtricitabine DMF submitted to regulatory agencies in the US is known as a USDMF. Emtricitabine USDMF includes data on Emtricitabine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Emtricitabine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Emtricitabine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Emtricitabine Drug Master File in Japan (Emtricitabine JDMF) empowers Emtricitabine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Emtricitabine JDMF during the approval evaluation for pharmaceutical products. At the time of Emtricitabine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Emtricitabine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Emtricitabine Drug Master File in Korea (Emtricitabine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Emtricitabine. The MFDS reviews the Emtricitabine KDMF as part of the drug registration process and uses the information provided in the Emtricitabine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Emtricitabine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Emtricitabine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Emtricitabine suppliers with KDMF on PharmaCompass.
A Emtricitabine written confirmation (Emtricitabine WC) is an official document issued by a regulatory agency to a Emtricitabine manufacturer, verifying that the manufacturing facility of a Emtricitabine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Emtricitabine APIs or Emtricitabine finished pharmaceutical products to another nation, regulatory agencies frequently require a Emtricitabine WC (written confirmation) as part of the regulatory process.
click here to find a list of Emtricitabine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Emtricitabine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Emtricitabine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Emtricitabine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Emtricitabine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Emtricitabine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Emtricitabine suppliers with NDC on PharmaCompass.
Emtricitabine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Emtricitabine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Emtricitabine GMP manufacturer or Emtricitabine GMP API supplier for your needs.
A Emtricitabine CoA (Certificate of Analysis) is a formal document that attests to Emtricitabine's compliance with Emtricitabine specifications and serves as a tool for batch-level quality control.
Emtricitabine CoA mostly includes findings from lab analyses of a specific batch. For each Emtricitabine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Emtricitabine may be tested according to a variety of international standards, such as European Pharmacopoeia (Emtricitabine EP), Emtricitabine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Emtricitabine USP).

