
Synopsis
Synopsis
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
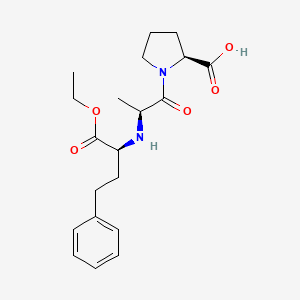

1. Enalapril Maleate
2. Maleate, Enalapril
3. Mk 421
4. Mk-421
5. Mk421
6. Renitec
7. Renitek
1. 75847-73-3
2. Vasotec
3. Enalaprilum
4. Enalaprila
5. Olinapril
6. Lexxel
7. Enalaprilum [inn-latin]
8. Enalaprila [inn-spanish]
9. Enalapril (inn)
10. Epaned
11. C09aa02
12. Chembl578
13. 1-(n-((s)-1-carboxy-3-phenylpropyl)-l-alanyl)-l-proline 1'-ethyl Ester
14. Bonuten
15. 69pn84io1a
16. Chebi:4784
17. (s)-1-(n-(1-(ethoxycarbonyl)-3-phenylpropyl)-l-alanyl)-l-proline
18. Ncgc00021569-04
19. Enalapril [inn]
20. ((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)-l-alanyl-l-proline
21. L-proline, N-[(1s)-1-(ethoxycarbonyl)-3-phenylpropyl]-l-alanyl-
22. Enalapril Richet
23. Dsstox_cid_2982
24. Dsstox_rid_76817
25. Dsstox_gsid_22982
26. (2s)-1-[(2s)-2-[[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]pyrrolidine-2-carboxylic Acid
27. (s)-1-{(s)-2-[1-((s)-ethoxycarbonyl)-3-phenyl-propylamino]-propionyl}-pyrrolidine-2-carboxylic Acid
28. Gadopril
29. Hsdb 6529
30. Enalapril (tn)
31. (2s)-1-[(2s)-2-{[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]pyrrolidine-2-carboxylic Acid
32. Cas-75847-73-3
33. Mk-421
34. Enalapril [inn:ban]
35. Analapril
36. Unii-69pn84io1a
37. Enaladex
38. L-proline, N-((1s)-1-(ethoxycarbonyl)-3-phenylpropyl)-l-alanyl-
39. Enalapril, Aldrichcpr
40. Spectrum_001307
41. Enalapril [mi]
42. Schembl18
43. N-[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]-l-alanyl-l-proline
44. Prestwick3_000314
45. Spectrum2_001455
46. Spectrum3_001478
47. Spectrum4_000008
48. Spectrum5_001107
49. Enalapril [vandf]
50. Enalapril Maleate Usp Xxi
51. Enalapril [mart.]
52. Enalapril [who-dd]
53. Bspbio_000308
54. Bspbio_003035
55. Kbiogr_000355
56. Kbioss_001787
57. Bidd:gt0751
58. Divk1c_000408
59. Spbio_001349
60. Bpbio1_000340
61. Gtpl6322
62. Dtxsid5022982
63. Kbio1_000408
64. Kbio2_001787
65. Kbio2_004355
66. Kbio2_006923
67. Kbio3_002535
68. Ninds_000408
69. Hms2090e08
70. Hy-b0331
71. N-[(2s)-1-ethoxy-1-oxo-4-phenyl-2-butanyl]-l-alanyl-l-proline
72. Zinc3791297
73. Tox21_110872
74. Bdbm50017129
75. Mfcd00865774
76. N-{(1s)-1-[(ethyloxy)carbonyl]-3-phenylpropyl}-l-alanyl-l-proline
77. Akos025310615
78. Tox21_110872_1
79. Db00584
80. Idi1_000408
81. Ncgc00016932-01
82. Ncgc00021569-05
83. Ncgc00021569-06
84. Ncgc00021569-09
85. Sbi-0051691.p002
86. Cas-76095-16-4
87. C06977
88. D07892
89. H10947
90. Ab00053615-17
91. Ab00053615-18
92. Ab00053615_19
93. Ab00053615_20
94. 847e733
95. A838525
96. A838609
97. Q422185
98. Brd-k57545991-050-16-5
99. N-(1(s)-ethoxycarbonyl-3-phenylpropyl)-l-alanyl-l-proline
100. N-[1-(s)-ethoxycarbonyl-3-phenylpropyl]-l-alanyl-l-proline
101. Nalpha -[(s)-1-ethoxycarbonyl-3-phenylpropyl]-l-alanyl-l-proline
102. L-proline, 1-(n-(1-(ethoxycarbonyl)-3-phenylpropyl)-l-alanyl)-, (s)-
103. (s)-1-(n-(1-(ethoxycarbonyl)-3-phenylpropyl)-l-alanyl)-l-proline;enalapril Maleate Salt
104. (sss)1-[2-(1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl]-pyrrolidine-2-carboxylic Acid
105. 1-[2-(1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl]-pyrrolidine-2-carboxylic Acid
106. (2s)-1-[(2s)-2-[[(1r)-1-ethoxycarbonyl-3-phenyl-propyl]amino]propanoyl]pyrrolidine-2-carboxylic Acid; Maleic Acid
107. (s)-1-((s)-2-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino)propanoyl)pyrrolidine-2-carboxylic Acid
108. (s)-1-((s)-2-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino)propanoyl)pyrrolidine-2-carboxylicacid
109. (s)-1-((s)-2-((r)-1-ethoxy-1-oxo-4-phenylbutan-2-ylamino)propanoyl)pyrrolidine-2-carboxylic Acid
110. (s)-1-[(r)-2-((r)-1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl]-pyrrolidine-2-carboxylic Acid
111. (s,s,s)-1-[2-(1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl]-pyrrolidine-2-carboxylic Acid
112. 1-[2-(1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl]-pyrrolidine-2-carboxylic Acid (enalapril)
113. 1-[2-(1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl]-pyrrolidine-2-carboxylic Acid(enalapril)
114. 2-[2-(1-carboxy-3-phenyl-propylamino)-propionyl]-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic Acid(enalapril)
1. Sodium Enalapril
2. Enalapril Sodium
| Molecular Weight | 376.4 g/mol |
|---|---|
| Molecular Formula | C20H28N2O5 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 10 |
| Exact Mass | 376.19982200 g/mol |
| Monoisotopic Mass | 376.19982200 g/mol |
| Topological Polar Surface Area | 95.9 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 519 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Angiotensin-Converting Enzyme Inhibitors; Antihypertensive Agents
National Library of Medicine's Medical Subject Headings. Enalapril. Online file (MeSH, 2017). Available from, as of August 30, 2017: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Enalapril is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 30, 2017: https://clinicaltrials.gov/
Enalapril maleate tablets are indicated for the treatment of hypertension. Enalapril maleate tablets are effective alone or in combination with other antihypertensive agents, especially thiazide-type diuretics. The blood pressure lowering effects of enalapril maleate tablets and thiazides are approximately additive. /Included in US product label/
NIH; DailyMed. Current Medication Information for Enalapril Maleate (Enalapril Maleate Tablet) (Updated: July 2017). Available from, as of October 27, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bad08ec8-7f9e-47b6-83b9-437e0191080f
Enalapril maleate tablets are indicated for the treatment of symptomatic congestive heart failure, usually in combination with diuretics and digitalis. /Included in US product label/
NIH; DailyMed. Current Medication Information for Enalapril Maleate (Enalapril Maleate Tablet) (Updated: July 2017). Available from, as of October 27, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bad08ec8-7f9e-47b6-83b9-437e0191080f
For more Therapeutic Uses (Complete) data for Enalapril (9 total), please visit the HSDB record page.
/BOXED WARNING/ When pregnancy is detected, discontinue enalapril maleate tablets as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for Enalapril Maleate (Enalapril Maleate Tablet) (Updated: July 2017). Available from, as of October 27, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bad08ec8-7f9e-47b6-83b9-437e0191080f
The most frequent adverse cardiovascular effect of enalapril or enalaprilat is hypotension (including postural hypotension and other orthostatic effects), which occurs in about 1-2% of patients with hypertension and in about 5-7% of those with heart failure, following an initial dose or during extended therapy. Syncope occurred in approximately 0.5 or 2% of patients with hypertension or heart failure, respectively. Hypotension or syncope has required discontinuance of therapy in about 0.1 or 2% of patients with hypertension or heart failure, respectively, receiving enalapril.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2085
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue enalapril maleate tablets as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
NIH; DailyMed. Current Medication Information for Enalapril Maleate (Enalapril Maleate Tablet) (Updated: July 2017). Available from, as of October 27, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bad08ec8-7f9e-47b6-83b9-437e0191080f
Angioedema may occur, especially following the first dose of enalapril, and, if associated with laryngeal edema, may be fatal. If laryngeal stridor or angioedema of the face, extremities, lips, tongue, or glottis occurs, enalapril should be discontinued and the patient carefully observed until swelling disappears. If swelling is confined to the face and lips, the condition generally responds without treatment; however, antihistamines may provide symptomatic relief. Swelling of the tongue, glottis, or larynx may cause airway obstruction, and appropriate therapy (eg, epinephrine, maintenance of patent airway) should be initiated immediately. Patients should be informed that swelling of the face, eyes, lips, or tongue or difficulty in breathing may be signs and symptoms of angioedema, and that they should discontinue enalapril and notify their physician immediately if any of these conditions occurs. The possibility that patients with a history of angioedema unrelated to angiotensin converting enzyme inhibitors may be at increased risk of developing angioedema while receiving the drugs should be considered. Enalapril is contraindicated in patients with a history of angioedema related to angiotensin converting enzyme inhibitor therapy. Enalapril also is contraindicated in patients with known hypersensitivity to the drug or any ingredient in the formulation.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2087
For more Drug Warnings (Complete) data for Enalapril (28 total), please visit the HSDB record page.
Indicated for the management of essential or renovascular hypertension as monotherapy or in combination with other antihypertensive agents, such as thiazide diuretics, for an additive effect. Indicated for the treatment of symptomatic congestive heart failure, usually in combination with diuretics and digitalis. Indicated for the management of asymptomatic left ventricular dysfunction in patients with an ejection fraction of to 35 percent to decrease the rate of development of overt heart failure and the incidence of hospitalization for heart failure.
FDA Label
Enalapril is an antihypertensive agent that exhibits natriuretic and uricosuric properties. Enalapril lowers blood pressure in all grades of essential and renovascular hypertension, and peripheral vascular resistance without causing an increase in heart rate. Individuals with low-renin hypertensive population were still responsive to enalapril. The duration of hypertensive effect in the systolic and diastolic blood pressure persists for at least 24 hours following initial administration of a single oral dose, and repeated daily administration of enalapril confers an additional reduction in blood pressure and a steady-state antihypertensive response may take several weeks. In patients with severe congestive heart failure and inadequate clinical response to conventional antihypertensive therapies, treatment with enalapril resulted in improvements in cardiac performance as observed by a reduction in both preload and afterload, and improved clinical status long-term. Furthermore, enalapril was shown to increase cardiac output and stroke volume while decreasing pulmonary capillary wedge pressure in patients with congestive heart failure refractory to conventional treatment with digitalis and diuretics. In clinical studies, enalapril reduced left ventricular mass, and did not affect cardiac function or myocardial perfusion during exercise. Enalapril is not highly associated with the risk of bradycardia unlike most diuretics and beta-blockers and it does not produce rebound hypertension upon discontinuation of therapy. Enalapril is not reported to produce hypokalaemia, hyperglycaemia, hyperuricaemia or hypercholesterolaemia. In the kidneys, enalapril was shown to increase renal blood flow and decrease renal vascular resistance. It also augmented the glomerular filtration rate in patients with a glomerular filtration rate less than 80 mL/min. When used in combination, enalapril was shown to attenuate the extent of drug-induced hypokalemia caused by hydrochlorothiazide and the antihypertensive effects of both drugs were potentiated.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
C09AA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09A - Ace inhibitors, plain
C09AA - Ace inhibitors, plain
C09AA02 - Enalapril
Absorption
Following oral administration, the peak plasma concentrations (Cmax) of enalapril is achieved within 1 hour post dosing while the Cmax of enalaprilat occurs at three to four hours post dosing. The steady-state is achieved by the fourth daily dose and there is no accumulation with repeated dosing. However, accumulation of enalaprilat may occur in patients with creatinine clearance less than 30 mL/min. Food intake is reported to have a minimal effect on drug absorption. Following oral administration, about 60% of enalapril was absorbed. Bioavailability of enalapril averaged about 40% when intravenous enalaprilat was used as a reference standard.
Route of Elimination
Enalapril is mainly eliminated through renal excretion, where approximately 94% of the total dose is excreted via urine or feces as either enalaprilat or unchanged parent compound. About 61% and 33% of the total dose can be recovered in the urine and feces, respectively. In the urine, about 40% of the recovered dose is in the form of enalaprilat.
Volume of Distribution
The volume of distribution of enalapril has not been established. Enalaprilat is shown to penetrate into most tissuesm, in particular the kidneys and vascular tissuem, although penetration of the blood-brain barrier has not been demonstrated after administration at therapeutic doses. In dog studies, enalapril and enalaprilat cross the blood-brain barrier poorly. Minimal penetration occurs into breast milk but significant fetal transfer occurs. The drug crosses the placental barrier in rats and hamsters.
Clearance
Following oral administration in healthy male volunteers, the renal clearance was approximately 158 47 mL/min. It is reported that enalapril and enalaprilat are undetectable in the plasma by 4 hours post-dosing.
Pharmacokinetic and pharmacodynamic of IV enalapril at 0.50 mg/kg, PO placebo and PO enalapril at three different doses (0.50, 1.00 and 2.00 mg/kg) were analyzed in 7 healthy horses. Serum concentrations of enalapril and enalaprilat were determined for pharmacokinetic analysis. Angiotensin-converting enzyme (ACE) activity, serum ureic nitrogen (SUN), creatinine and electrolytes were measured, and blood pressure was monitored for pharmacodynamic analysis. The elimination half-lives of enalapril and enalaprilat were 0.67 and 2.76 hr respectively after IV enalapril. Enalapril concentrations after PO administrations were below the limit of quantification (10 ng/mL) in all horses and enalaprilat concentrations were below the limit of quantification in 4 of the 7 horses. Maximum mean ACE inhibitions from baseline were 88.38, 3.24, 21.69, 26.11 and 30.19% for IV enalapril at 0.50 mg/kg, placebo and PO enalapril at 0.50, 1.00 and 2.00 mg/kg, respectively. Blood pressures, SUN, creatinine and electrolytes remained unchanged during the experiments.
PMID:24972864 Gomez-Diez M et al; Res Vet Sci 97 (1): 105-10 (2014)
Enalapril maleate, unlike enalaprilat, is well absorbed following oral administration. Although enalaprilat is a more potent angiotensin converting enzyme inhibitor than enalapril, it is poorly absorbed from the GI tract because of its high polarity, with only about 3-12% of an orally administered dose being absorbed. Approximately 55-75% of an oral dose of enalapril maleate is rapidly absorbed from the GI tract in healthy individuals and hypertensive patients. Food does not appear to substantially affect the rate or extent of absorption of enalapril maleate. Following oral administration, enalapril maleate appears to undergo first pass metabolism principally in the liver, being hydrolyzed to enalaprilat.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2090
The hypotensive effect of a single oral dose of enalapril maleate is usually apparent within 1 hr and maximal in 4-8 hr. The hypotensive effect of usual doses of the drug generally persists for 12-24 hr but may diminish toward the end of the dosing interval in some patients. Reduction in blood pressure may be gradual, and several weeks of therapy may be required before the full effect is achieved.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2090
Following IV administration of enalaprilat, the hypotensive effect is usually apparent within 5-15 min with maximal effect occurring within 1-4 hr; the duration of hypotensive effect appears to be dose related, but with the recommended doses, the duration of action in most patients is approximately 6 hr. Plasma angiotensin converting enzyme inhibition and reduction in blood pressure appear to be correlated to a plasma enalaprilat concentration of 10 ng/mL, a concentration at which maximal blockade of plasma angiotensin converting enzyme is achieved. After withdrawal of enalapril or enalaprilat, blood pressure gradually returns to pretreatment levels; rebound hypertension following abrupt withdrawal of the drug has not been reported to date. /Enalaprilat/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2090
For more Absorption, Distribution and Excretion (Complete) data for Enalapril (11 total), please visit the HSDB record page.
About 60% of the absorbed dose is extensively hydrolyzed to enalaprilat via de-esterification mediated by hepatic esterases. In humans, metabolism beyond bioactivation to enalaprilat is not observed.
About 60% of an absorbed dose of enalapril is extensively hydrolyzed to enalaprilat, principally in the liver via esterases. About 20% appears to be hydrolyzed on first pass through the liver; this hydrolysis does not appear to occur in plasma in humans. Enalaprilat is a more potent angiotensin converting enzyme inhibitor than enalapril. There is no evidence of other metabolites of enalapril in humans, rats, or dogs. However, a despropyl metabolite of enalaprilat was identified in urine in rhesus monkeys, accounting for 13% of an oral dose of enalapril maleate. Hydrolysis of enalapril to enalaprilat may be delayed and/or impaired in patients with severe hepatic impairment, but the pharmacodynamic effects of the drug do not appear to be significantly altered.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2090
The average terminal half life of enalaprilat is 35-38 hours. The effective half life following multiple doses is 11-14 hours. The prolonged terminal half-life is due to the binding of enalaprilat to ACE.
Following oral admin, the half-life of unchanged enalapril appears to be <2 hr in healthy individuals and in patients with normal hepatic and renal functions, but may be increased in patients with congestive heart failure. Following oral admin of a single 5 or 10 mg dose of enalapril maleate in patients with congestive heart failure, the half-life of enalapril was 3.4 or 5.8 hr, respectively.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2090
Elimination of enalaprilat may also be prolonged in patients with congestive heart failure or impaired hepatic function compared with healthy individuals and patients with hypertension observations of serum concns of enalaprilat over long periods following oral or iv admin suggest that enalaprilat has an avg terminal half-life of about 35-38 hr (range: 30-87 hr). ...The effective half-life for accumulation of enalaprilat (determined from urinary recovery) has been reported to average about 11 hr in healthy individuals with normal renal function.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2090
The renin-angiotensin-aldosterone system (RAAS) is a signaling pathway that works in synergism with the sympathetic system to regulate blood pressure and fluid and electrolyte homeostasis. Activation of this system upon stimulation by different factors, such as low blood pressure and nerve impulses, leads to increased release of norepinephrine (NE) from sympathetic nerve terminals and effects on the vascular growth, vasoconstriction, and salt retention in the kidneys. Renin is released from Renin acts on the precursor prottein angiotensinogen, which is a plasma globulin synthesized from the liver, to produce cleaved peptide hormone angiotensin I. Angiotensin I then can be further cleaved by ACE to produce angiotensin II, a vasoconstrictive peptide hormone. Present in different isoforms, angiotensin converting enzyme (ACE) is peptidyl dipeptidase enzyme expressed in various tissues, including the vascular tissues, such as the heart, brain, and kidneys. ACE also plays a role in inactivation of bradykinin, a potent vasodepressor peptide. Angiotensin II mediates various actions on the body by working on its G-protein coupled receptors, AT1 and AT2. It causes direct vasoconstriction of precapillary arterioles and postcapillary venules, inhibits the reuptake of NE thereby increasing available levels, stimulates the release of catecholamines from the adrenal medulla, reduces urinary excretion of sodium ions and water by promoting proximal tubular reabsorption, stimulates synthesis and release of aldosterone from the adrenal cortex, and stimulates hypertrophy of both vascular smooth muscle cells and cardiac myocytes. Enalapril is a pharmacologically inactive prodrug that requires hepatic biotransformation to form [enalaprilat], its active metabolite that works on the RAAS to inhibit ACE. Biotransformation is critial for the therapeutic actions of the drug, as enalapril itself is only a weak inhibitor of ACE. ACE inhibition results in reduced production and plasma levels of angiotensin II, increased plasma renin activity due to the loss of feedback inhibition by angiotensin II, and decreased aldosterone secretion. However, plasma aldosterone levels usually return to normal during long-term administration of enalapril. Decreased levels of angiotensin II subsequently leads to the dilatation of peripheral vessles and reduced vascular resistance which in turn lower blood pressure. While inhibition of ACE leading to suppression of RAAS is thought to be the primary mechanism of action of enalapril, the drug was shown to still exert antihypertensive effects on individuals with low-renin hypertension. It is suggested that enalapril may mediate its pharmacological actions via other modes of action that are not fully understood. As ACE is structurally similar to kininase I, which is a carboxypeptidase that degrades bradykinin, whether increased levels of bradykinin play a role in the therapeutic effects of enalapril remains to be elucidated.
Enalapril maleate is a prodrug of enalaprilat and has little pharmacologic activity until hydrolyzed in vivo to enalaprilat. ... Enalapril prevents the conversion of angiotensin I to angiotensin II (a potent vasoconstrictor) through inhibition of angiotensin-converting enzyme (ACE). The drug competes with physiologic substrate (angiotensin I) for the active site of ACE; the affinity of enalaprilat for ACE is approximately 200,000 times greater than that of angiotensin I. In vitro on a molar basis, the affinity of enalaprilat for ACE is 300-1000 or 2-17 times that of enalapril or captopril, respectively. However, in vitro on a molar basis, the ACE-inhibitory effect of enalapril was shown to be similar to that of enalaprilat in rat plasma and kidneys, because these tissues extensively hydrolyze enalapril to form enalaprilat. The drug apparently does not inhibit brain ACE in animals.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2089

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
58
PharmaCompass offers a list of Enalapril Maleate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Enalapril Maleate manufacturer or Enalapril Maleate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Enalapril Maleate manufacturer or Enalapril Maleate supplier.
PharmaCompass also assists you with knowing the Enalapril Maleate API Price utilized in the formulation of products. Enalapril Maleate API Price is not always fixed or binding as the Enalapril Maleate Price is obtained through a variety of data sources. The Enalapril Maleate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Enalapril manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Enalapril, including repackagers and relabelers. The FDA regulates Enalapril manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Enalapril API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Enalapril manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Enalapril supplier is an individual or a company that provides Enalapril active pharmaceutical ingredient (API) or Enalapril finished formulations upon request. The Enalapril suppliers may include Enalapril API manufacturers, exporters, distributors and traders.
click here to find a list of Enalapril suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Enalapril DMF (Drug Master File) is a document detailing the whole manufacturing process of Enalapril active pharmaceutical ingredient (API) in detail. Different forms of Enalapril DMFs exist exist since differing nations have different regulations, such as Enalapril USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Enalapril DMF submitted to regulatory agencies in the US is known as a USDMF. Enalapril USDMF includes data on Enalapril's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Enalapril USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Enalapril suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Enalapril Drug Master File in Japan (Enalapril JDMF) empowers Enalapril API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Enalapril JDMF during the approval evaluation for pharmaceutical products. At the time of Enalapril JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Enalapril suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Enalapril Drug Master File in Korea (Enalapril KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Enalapril. The MFDS reviews the Enalapril KDMF as part of the drug registration process and uses the information provided in the Enalapril KDMF to evaluate the safety and efficacy of the drug.
After submitting a Enalapril KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Enalapril API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Enalapril suppliers with KDMF on PharmaCompass.
A Enalapril CEP of the European Pharmacopoeia monograph is often referred to as a Enalapril Certificate of Suitability (COS). The purpose of a Enalapril CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Enalapril EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Enalapril to their clients by showing that a Enalapril CEP has been issued for it. The manufacturer submits a Enalapril CEP (COS) as part of the market authorization procedure, and it takes on the role of a Enalapril CEP holder for the record. Additionally, the data presented in the Enalapril CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Enalapril DMF.
A Enalapril CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Enalapril CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Enalapril suppliers with CEP (COS) on PharmaCompass.
A Enalapril written confirmation (Enalapril WC) is an official document issued by a regulatory agency to a Enalapril manufacturer, verifying that the manufacturing facility of a Enalapril active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Enalapril APIs or Enalapril finished pharmaceutical products to another nation, regulatory agencies frequently require a Enalapril WC (written confirmation) as part of the regulatory process.
click here to find a list of Enalapril suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Enalapril as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Enalapril API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Enalapril as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Enalapril and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Enalapril NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Enalapril suppliers with NDC on PharmaCompass.
Enalapril Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Enalapril GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Enalapril GMP manufacturer or Enalapril GMP API supplier for your needs.
A Enalapril CoA (Certificate of Analysis) is a formal document that attests to Enalapril's compliance with Enalapril specifications and serves as a tool for batch-level quality control.
Enalapril CoA mostly includes findings from lab analyses of a specific batch. For each Enalapril CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Enalapril may be tested according to a variety of international standards, such as European Pharmacopoeia (Enalapril EP), Enalapril JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Enalapril USP).
