

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
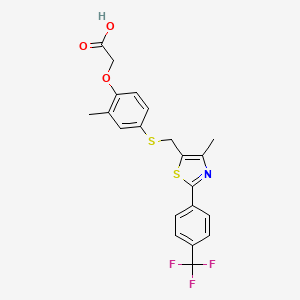

1. Cardarine
2. Gw 1516
3. Gw 501516
4. Gw-1516
5. Gw-501516
6. Gw1516
7. Gw501516
1. 317318-70-0
2. Gw501516
3. Gw 501516
4. Gw-501516
5. Gsk-516
6. Cardarine
7. Gw1516
8. Gw 1516
9. Unii-7i2ha1nu22
10. Gw-1516
11. 2-(4-((2-(4-(trifluoromethyl)phenyl)-4-methylthiazol-5-yl)methylthio)-2-methylphenoxy)acetic Acid
12. 2-(2-methyl-4-(((4-methyl-2-(4-(trifluoromethyl)phenyl)thiazol-5-yl)methyl)thio)phenoxy)acetic Acid
13. 2-[2-methyl-4-[[4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl]methylsulfanyl]phenoxy]acetic Acid
14. Gw-516
15. Chembl38943
16. 7i2ha1nu22
17. {2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl}methyl)sulfanyl]phenoxy}acetic Acid
18. Chebi:73726
19. 2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl}methyl)sulfanyl]phenoxy}acetic Acid
20. Gw501,516
21. Gw 501,516
22. Gw-501,516
23. Gsk 516
24. Gw501516 (endurobol)
25. Schembl68714
26. 2-[2-methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]acetic Acid
27. Mls006010753
28. Endurobol;gw501516
29. Gtpl2687
30. Dtxsid3041037
31. Bdbm28661
32. Ex-a723
33. Hms3674c03
34. Hms3744o09
35. Bcp21491
36. Zinc1549989
37. Mfcd09033000
38. Pdsp1_000255
39. Pdsp2_000254
40. S5615
41. Akos015965103
42. Ccg-269268
43. Cs-0438
44. Db05416
45. Ex-3881
46. Sb19569
47. Gw501516(g)
48. Gw501516(gsk-516)
49. Ncgc00241455-01
50. Ncgc00241455-02
51. Ncgc00241455-08
52. Ac-23001
53. Hy-10838
54. Smr004701706
55. Am20040157
56. A26949
57. Gw501516, >=98% (hplc)
58. 318g700
59. 6t-0058
60. Benzenesulfonylchloride,4-(2-methylpropyl)-(9ci)
61. J-018519
62. Q5515069
63. Brd-k14880289-001-01-4
64. (2-methyl-4-(((4-methyl-2-(4-(trifluoromethyl)phenyl)-1,3-thiazol-5-yl)methyl)thio)phenoxy)acetic Acid
65. {2-methyl-4-{{4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl}methylthio}phenoxy}acetic Acid, Analytical Standard
66. {4-[({4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl}methyl)sulfanyl]-2-methylphenoxy}acetic Acid
67. 2-(2-methyl-4-((5-methyl-2-(4-(trifluoromethyl)phenyl)thiazol-4-yl)methylthio)phenoxy)acetic Acid;gw 501516
68. 2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazole-5-yl}methyl)sulfanyl]phenoxy}acetic Acid
69. 7t1
70. Acetic Acid, (2-methyl-4-(((4-methyl-2-(4-(trifluoromethyl)phenyl)-5-thiazolyl)methyl)thio)phenoxy)-
71. Acetic Acid, 2-[2-methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]-
| Molecular Weight | 453.5 g/mol |
|---|---|
| Molecular Formula | C21H18F3NO3S2 |
| XLogP3 | 5.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 453.06802027 g/mol |
| Monoisotopic Mass | 453.06802027 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 572 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in hyperlipidemia.
This drug regulates fatty acid oxidation in several tissues, such as skeletal muscle and adipose tissue. Overexpression of PPARdelta using a transgenic murine model promotes an increase of muscle oxidative capability. It also plays a major role in the metabolic adaptations to western diet characterized by an excessive amount of saturated fat.
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
