
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
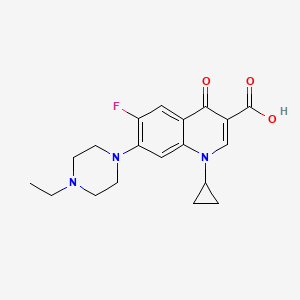

1. Bay Vp 2674
2. Bay-vp-2674
3. Baytril
4. Endrofloxicin
1. 93106-60-6
2. Baytril
3. Enrofloxacine
4. Cfpq
5. Bay Vp 2674
6. Enrofloxacino
7. Enrofloxacinum
8. 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
9. Enroxil
10. Baytril (tn)
11. N-ethylciprofloxacin
12. Erfx
13. 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic Acid
14. 3-quinolinecarboxylic Acid, 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-
15. Mfcd00792463
16. 3dx3xek1bn
17. Nsc-758616
18. Enrofloxacin For Veterinary Use
19. Mls000069441
20. Chebi:35720
21. Bay Vp 2674;pd160788
22. Endrofloxicin
23. Ncgc00018143-04
24. Cpd000059011
25. Pd160788
26. Smr000059011
27. 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxoquinoline-3-carboxylic Acid
28. Dsstox_cid_25619
29. Dsstox_rid_81007
30. Dsstox_gsid_45619
31. Enrofloxacine [french]
32. Enrofloxacinum [latin]
33. Enrofloxacino [spanish]
34. Bay-vp-2674
35. Cas-93106-60-6
36. Enrofloxacin [usan:ban:inn]
37. Hsdb 6952
38. Unii-3dx3xek1bn
39. Brn 5307824
40. Ccris 8214
41. Enrofloxacin [usan:usp:inn:ban]
42. Enrofloxacin-[d5]
43. Opera_id_1106
44. Enrofloxacin (usp/inn)
45. Enrofloxacin [mi]
46. Enrofloxacin (usan/inn)
47. Enrofloxacin [inn]
48. Enrofloxacin [hsdb]
49. Enrofloxacin [usan]
50. 1,4-dihydro-1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-4-oxo-3-quinolinecarboxylic Acid
51. Bay-vp2674
52. Enrofloxacin [mart.]
53. Mls001076496
54. Mls001424169
55. Chembl15511
56. Enrofloxacin [usp-rs]
57. Schembl149150
58. Spectrum1503721
59. Dtxsid1045619
60. Enrofloxacin [green Book]
61. Hms2052o09
62. Hms2090e12
63. Hms2093i21
64. Hms2234m11
65. Hms3373p14
66. Hms3394o09
67. Hms3715b18
68. Pharmakon1600-01503721
69. Zinc597112
70. Enrofloxacin [usp Impurity]
71. Albb-030792
72. Bcp15457
73. Hy-b0502
74. Enrofloxacin [usp Monograph]
75. Enrofloxacin, >=98.0% (hplc)
76. Tox21_110831
77. Bbl009982
78. Dl-384
79. Nsc758616
80. S3059
81. Stk711109
82. Akos005530685
83. Bay-vp2674;pd160788
84. Tox21_110831_1
85. Ac-7614
86. Ccg-101102
87. Db11404
88. Ks-5010
89. Nc00352
90. Nsc 758616
91. 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxo-quinoline-3-carboxylic Acid
92. 1-cyclopropyl-7-(4-ethylpiperazinyl)-6-fluoro-4-oxohydroquinoline-3-carboxylic Acid
93. Enrofloxacin 100 Microg/ml In Methanol
94. Ncgc00018143-01
95. Ncgc00018143-02
96. Ncgc00018143-03
97. Ncgc00018143-05
98. Ncgc00021632-03
99. 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic Acid
100. 3-quinolinecarboxylic Acid, 1,4-dihydro-1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-4-oxo-
101. Enrofloxacin 1000 Microg/ml In Methanol
102. Sbi-0206725.p001
103. Db-057368
104. B1742
105. E0786
106. Ft-0625663
107. Ft-0667862
108. D02473
109. Ab00384269-16
110. Ab00384269_17
111. Ab00384269_18
112. Enrofloxacin, Vetranal(tm), Analytical Standard
113. 106e606
114. A844445
115. Q414413
116. Sr-01000000045
117. Sr-01000000045-3
118. Brd-k76534306-001-11-0
119. Enrofloxacin For Veterinary Use [ep Impurity]
120. Enrofloxacin For Veterinary Use [ep Monograph]
121. Enrofloxacin, European Pharmacopoeia (ep) Reference Standard
122. Enrofloxacin, United States Pharmacopeia (usp) Reference Standard
123. 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-4-oxo-3-quinolinecarboxylic Acid
124. Enrofloxacin For System Suitability, European Pharmacopoeia (ep) Reference Standard
125. Enrofloxacin, Pharmaceutical Secondary Standard; Certified Reference Material
126. 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-3-quinoline-carboxylic Acid
127. 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-3-quinolinecarboxylic Acid
128. 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3- Quinolonecarboxylic Acid
129. 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic Acid, 9ci
130. 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoranyl-4-oxidanylidene-quinoline-3-carboxylic Acid
| Molecular Weight | 359.4 g/mol |
|---|---|
| Molecular Formula | C19H22FN3O3 |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 359.16451973 g/mol |
| Monoisotopic Mass | 359.16451973 g/mol |
| Topological Polar Surface Area | 64.1 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 613 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Pharmacokinetics and bioavailability of enrofloxacin were determined after single intravenous (IV) and intramuscular (IM) administrations of 5 mg/kg body weight (BW) to 5 healthy adult Angora goats. Plasma enrofloxacin concentrations were measured by high performance liquid chromatography. Pharmacokinetics were best described by a 2-compartment open model. The elimination half-life and volume of distribution after IV and IM administrations were similar (t1/2beta, 4.0 to 4.7 hr and Vd(ss),1.2 to 1.5 L/kg, respectively). Enrofloxacin was rapidly (t1/2a, 0.25 hr) and almost completely absorbed (F, 90%) after IM administration. Mean plasma concentrations of enrofloxacin at 24 hr after IV and IM administration (0.07 and 0.09 microg/mL, respectively) were higher than the minimal inhibitory concentration (MIC) values for most pathogens. In conclusion, once-daily IV and IM administration of enrofloxacin (5 mg/kg BW) in Angora goats may be useful in treatment of infectious diseases caused by sensitive pathogens.
PMID:11227198 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1189645 Elmas M et al; Can J Vet Res 65 (1): 64-7 (2001)
Plasma, urine, and skin drug concentrations were determined for dogs (n=12) given five daily oral doses of marbofloxacin (MAR) (2.75 mg/kg), enrofloxacin (ENR) (5.0 mg/kg) or difloxacin (DIF) (5.0 mg/kg). Concentrations of the active metabolite of ENR, ciprofloxacin (CIP), were also determined. The three-period, three-treatment crossover experimental design included a 21-day washout period between treatments. Area under the plasma drug concentration vs. time curve (AUC0-last, microg/mlxhr of MAR was greater than for ENR, CIP, ENR/CIP combined, and DIF. Maximum concentration (Cmax) of MAR was greater than ENR, CIP, and DIF. Time of maximum plasma concentration (Tmax) was similar for MAR and DIF; Tmax occurred earlier for ENR and later for CIP. Plasma half-life (t1/2) of MAR was longer than for ENR, CIP, and DIF. Urine concentrations of DIF were less than MAR or ENR/CIP combined, but urine concentrations of MAR and ENR/CIP combined did not differ. DIF skin concentrations were less than the concentrations of MAR or ENR/CIP combined 2 h after dosing, but skin concentrations of MAR and ENR/CIP combined did not differ.
PMID:11107003 Frazier DL et al; J Vet Pharmacol Ther 23 (5): 293-302 (2000)
Serum concentrations and pharmacokinetics of enrofloxacin were studied in 6 mares after intravenous (IV) and intragastric (IG) administration at a single dose rate of 7.5 mg/kg body weight. In experiment 1, an injectable formulation of enrofloxacin (100 mg/ml) was given IV. At 5 min after injection, mean serum concentration was 9.04 microg/mL and decreased to 0.09 microg/mL by 24 hr. Elimination half-life was 5.33 +/- 1.05 hr and the area under the serum concentration vs time curve (AUC) was 21.03 +/- 5.19 mg x hr/L. In experiment 2, the same injectable formulation was given IG. The mean peak serum concentration was 0.94 +/- 0.97 microg/ml at 4 hr after administration and declined to 0.29 +/- 0.12 microg/ml by 24 hr. Absorption of this enrofloxacin preparation after IG administration was highly variable, and for this reason, pharmacokinetic values for each mare could not be determined. In experiment 3, a poultry formulation (32.3 mg/ml) was given IG. The mean peak serum concentration was 1.85 +/- 1.47 microg/ml at 45 min after administration and declined to 0.19 +/- 0.06 microg/mL by 24 h. Elimination half-life was 10.62 +/- 5.33 h and AUC was 16.30 +/- 4.69 mg x h/L. Bioavailability was calculated at 78.29 +/- 16.55%. Minimum inhibitory concentrations of enrofloxacin were determined for equine bacterial culture specimens submitted to the microbiology laboratory over an 11-month period. The minimum inhibitory concentration of enrofloxacin required to inhibit 90% of isolates (MIC90) was 0.25 microg/ml for Staphylococcus aureus, Escherichia coli, Salmonella spp., Klebsiella spp., and Pasteurella spp. The poultry formulation was well tolerated and could be potentially useful in the treatment of susceptible bacterial infections in adult horses. The injectable enrofloxacin solution should not be used orally.
PMID:10935883 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1189609 Haines GR et al; Can J Vet Res 64 (3): 171-7 (2000)
Concentrations of enrofloxacin equivalent activity were determined by microbiological assay in the plasma of healthy and E. coli-infected broilers following single intravenous and oral administrations at 10 mg/kg. Tissue distribution and residue-depletion following multiple oral doses (10 mg/kg for 3 successive days) were investigated. Pharmacokinetic variables were determined using compartmental and non-compartmental analytical methods. Plasma enrofloxacin concentrations after intravenous dosing to healthy and infected birds were best described by a two-compartments model. Enrofloxacin concentrations in plasma of infected birds were lower than those of healthy ones. The disposition kinetics of intravenously administered drug in healthy and infected birds were somewhat different. The elimination half-life (t1/2 beta) was 4.75 vs. 3.63 hr; mean residence time (MRT) was 6.72 vs 4.90 hr; apparent volume of the central compartment (Vc) was 1.11 vs 1.57 l/kg; rate constant for transfer from peripheral to central compartment (k21) was 1.15 vs 1.41 hr-1 and total body clearance (ClB) was 0.35 vs 0.53 l/hr/kg in healthy and infected birds, respectively. After oral administration, the absorption half-life (t1/2abs) in the infected birds was significantly longer than in healthy birds, while elimination half-life (t1/2el) and MRT were significantly shorter. Bioavailability was higher in infected birds (72.50%) as compared to healthy ones (69.78%). Enrofloxacin was detected in the tissues of healthy and infected birds after daily oral dosing of 10 mg/kg for 3 days. It was more concentrated in liver, kidney, and breast muscle. The minimal inhibitory concentration (MIC) of enrofloxacin against E. coli was 0.064 microgram/ml. On the basis of maintaining enrofloxacin plasma concentrations over the MIC, a dose of 10 mg/kg given intravenously every 20.14 hr or orally every 20.86 hr should provide tissue concentrations effective against E. coli infection in chickens.
PMID:10689795 Soliman GA; Dtsch Tierarztl Wochenschr 107 (1): 23-7 (2000)
For more Absorption, Distribution and Excretion (Complete) data for ENROFLOXACIN (6 total), please visit the HSDB record page.
The pharmacokinetics of enrofloxacin and its active metabolite ciprofloxacin were investigated in goats after a single intramuscular administration of enrofloxacin at 2.5 mg/kg body weight. The plasma concentrations of enrofloxacin and ciprofloxacin were determined simultaneously by a HPLC method. The peak concentrations (Cmax) of enrofloxacin (1.13 microg/ml) and ciprofloxacin (0.24 microg/ml) were observed at 0.8 and 1.2 hr, respectively. The elimination half-life (t1/2beta), volume of distribution (Vd(area)), total body clearance (Cl(B)) and mean residence time (MRT) of enrofloxacin were 0.74 hr, 1.42 l/kg, 1329 ml/hr per kg and 1.54 hr, respectively. The t1/2beta, area under the plasma concentration-time curve (AUC) and the MRT of ciprofloxacin were 1.38 h, 0.74 microg h/ml and 2.73 h, respectively. The metabolic conversion of enrofloxacin to ciprofloxacin was appreciable (36%) and the sum of the plasma concentrations of enrofloxacin and ciprofloxacin was maintained at or above 0.1 microg/ml for up to 4 hr. Enrofloxacin appears to be useful for the treatment of goat diseases associated with pathogens sensitive to this drug.
PMID:11334149 Rao GS et al; Vet Res Commun 25 (3): 197-204 (2001)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Controlled & Modified Release
Excipient Details : ACTILLETS are microcrystalline cellulose spheres developed using advanced drug delivery technology to enable effective loading & coating of particles.
Pharmacopoeia Ref : NA
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Capsule
Grade : Oral (Pharma Grade)
Application : Fillers, Diluents & Binders
Excipient Details : KoVidone® K25 is used as a low viscosity wet binder in solid dosage forms such as capsules and tablets.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : Microlose (Lactose Monohydrate & Microcrystalline Cellulose) is used as a diluent in oral dosage forms such as tablets.
Pharmacopoeia Ref : DMF, EXCiPAT, KOSHER, HALAL, W...
Technical Specs : Lactose Monohydrate – 40%, Microcrystalline cellulose – 60%
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : ProBlend (SMCC) is a co-processed excipient consists of microcrystalline cellulose & colloidal silicon dioxide, used as a diluent & binder in OSDs.
Pharmacopoeia Ref : USP-NF, DMF, EXCiPAT, KOSHER, ...
Technical Specs : NA
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Capsule
Grade : Oral
Application : Disintegrants & Superdisintegrants
Excipient Details : Sallyso (Croscarmellose Sodium) is a cross-linked polymer of carboxymethylcellulose sodium, used as a superdisintegrant in tablets and capsules.
Pharmacopoeia Ref : USP-NF, BP, IP, EP, DMF, EXCiP...
Technical Specs : Sallyso 0.5 to 3.0%
Ingredient(s) : Croscarmellose Sodium
Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : Starlose P40 (Starch & Lactose Monohydrate) is used as a diluent in oral dosage forms such as tablets.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Injectable / Parenteral
Grade : Parenteral
Application : Thickeners and Stabilizers
Excipient Details : L-Arginine is used as a cryoprotective agent and solution additive to stabilize proteins. It is used in injectable biologic & vaccine formulations.
Pharmacopoeia Ref : USP, EP, JP, ChP
Technical Specs : Low Endotoxin, Low Metals
Ingredient(s) : L-Arginine Excipient
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Not Available
Brand Name : Disintequik™ MCC 25
Application : Chewable & Orodispersible Aids
Excipient Details : Direct Tabletting Operations Where Fast Disintegration Is Required
Dosage Form : Capsule
Grade : Not Available
Brand Name : Sheffield™ Monohydrate ...
Application : Granulation
Excipient Details : Wet Granulation & Capsule Filling
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Not Available
Brand Name : Sheffield™ Monohydrate ...
Application : Fillers, Diluents & Binders
Excipient Details : Filler, Diluent, & Bulking Agent In A Wide Variety Of Pharmaceutical Tablets, Capsules, Powders, And Other Preparations
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Not Available
Brand Name : Sheffield™ Monohydrate ...
Application : Granulation
Excipient Details : Wet Granulation & Capsule Filling
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Not Available
Brand Name : Sheffield™ Monohydrate ...
Application : Granulation
Excipient Details : Wet Granulation & Capsule Filling
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Not Available
Brand Name : Sheffield™ Monohydrate ...
Application : Fillers, Diluents & Binders
Excipient Details : Filler, Diluent, & Bulking Agent In A Wide Variety Of Pharmaceutical Tablets, Capsules, Powders, And Other Preparations
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Not Available
Brand Name : Sheffield™ Monohydrate ...
Application : Fillers, Diluents & Binders
Excipient Details : Filler, Diluent, & Bulking Agent In A Wide Variety Of Pharmaceutical Tablets, Capsules, Powders, And Other Preparations
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Not Available
Brand Name : Sheffield™ Monohydrate ...
Application : Fillers, Diluents & Binders
Excipient Details : Filler, Diluent, & Bulking Agent In A Wide Variety Of Pharmaceutical Tablets, Capsules, Powders, And Other Preparations
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Not Available
Brand Name : Sheffield™ Spray Dried ...
Application : Direct Compression
Excipient Details : Pharmaceutical excipient used in direct compression
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Not Available
Brand Name : Sheffield™ Spray Dried ...
Application : Direct Compression
Excipient Details : Pharmaceutical excipient used in direct compression
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Brand Name : Croscarmellose Sodium
Application : Disintegrants & Superdisintegrants
Excipient Details : Cross Carmellose Sodium is fast disintegrating agent or a super-disintegrant used in pharmaceutical tablet formulations.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Croscarmellose Sodium
Dosage Form : Tablet
Grade : Oral
Brand Name : Microcrystalline Cellulos...
Application : Fillers, Diluents & Binders
Excipient Details : Microcrystalline Cellulose is most commonly used filler and binder in drug formulations, together with Lactose.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® LCM 120.s
Application : Granulation
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Sieved; Also Available as Microlex® LCM 180.s, Microlex® LCM 220.s
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® LCM 80.m
Application : Granulation
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Milled; Also Available as Microlex® LCM 180.m, Microlex® LCM 200.m
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® MCC 101
Application : Granulation
Excipient Details : Tablets made from these granules are typically easily disintegrated using conventional super disintegrates even when hard tablets are compressed.
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® MCC 102
Application : Direct Compression
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : Most popular excipient for the production of tablets and capsules. Offering an efficient and low dosage in capsules.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-10 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : Higher specific surface area and a smaller median particle size. This product is preferred for more critical and very fine herbal formulations.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-8-12 m2/g; Particle Size-5-9 µm
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-8 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : Meets Kosher and Halal preparations in Jewish and Arab cultures. Qualified in high specification standards requested by European formulators.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® PVD K30
Application : Solubilizers
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Also Available as Microlex® PVD K90.
Ingredient(s) : Povidone
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : TABCELL serves as an excellent excipient for solid dosage forms, providing numerous advantages for tablet formulations.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : TABLUBE is one of the oldest and most widely used lubricants for tablet, capsules and other solid dosage forms.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Dosage Form : Injectable / Parenteral
Grade : Oral, Parenteral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Capsule
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
19
PharmaCompass offers a list of Enrofloxacin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Enrofloxacin manufacturer or Enrofloxacin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Enrofloxacin manufacturer or Enrofloxacin supplier.
PharmaCompass also assists you with knowing the Enrofloxacin API Price utilized in the formulation of products. Enrofloxacin API Price is not always fixed or binding as the Enrofloxacin Price is obtained through a variety of data sources. The Enrofloxacin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Enrofloxacin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Enrofloxacin, including repackagers and relabelers. The FDA regulates Enrofloxacin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Enrofloxacin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Enrofloxacin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Enrofloxacin supplier is an individual or a company that provides Enrofloxacin active pharmaceutical ingredient (API) or Enrofloxacin finished formulations upon request. The Enrofloxacin suppliers may include Enrofloxacin API manufacturers, exporters, distributors and traders.
click here to find a list of Enrofloxacin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Enrofloxacin DMF (Drug Master File) is a document detailing the whole manufacturing process of Enrofloxacin active pharmaceutical ingredient (API) in detail. Different forms of Enrofloxacin DMFs exist exist since differing nations have different regulations, such as Enrofloxacin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Enrofloxacin DMF submitted to regulatory agencies in the US is known as a USDMF. Enrofloxacin USDMF includes data on Enrofloxacin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Enrofloxacin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Enrofloxacin suppliers with USDMF on PharmaCompass.
A Enrofloxacin CEP of the European Pharmacopoeia monograph is often referred to as a Enrofloxacin Certificate of Suitability (COS). The purpose of a Enrofloxacin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Enrofloxacin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Enrofloxacin to their clients by showing that a Enrofloxacin CEP has been issued for it. The manufacturer submits a Enrofloxacin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Enrofloxacin CEP holder for the record. Additionally, the data presented in the Enrofloxacin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Enrofloxacin DMF.
A Enrofloxacin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Enrofloxacin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Enrofloxacin suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Enrofloxacin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Enrofloxacin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Enrofloxacin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Enrofloxacin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Enrofloxacin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Enrofloxacin suppliers with NDC on PharmaCompass.
Enrofloxacin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Enrofloxacin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Enrofloxacin GMP manufacturer or Enrofloxacin GMP API supplier for your needs.
A Enrofloxacin CoA (Certificate of Analysis) is a formal document that attests to Enrofloxacin's compliance with Enrofloxacin specifications and serves as a tool for batch-level quality control.
Enrofloxacin CoA mostly includes findings from lab analyses of a specific batch. For each Enrofloxacin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Enrofloxacin may be tested according to a variety of international standards, such as European Pharmacopoeia (Enrofloxacin EP), Enrofloxacin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Enrofloxacin USP).

