

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
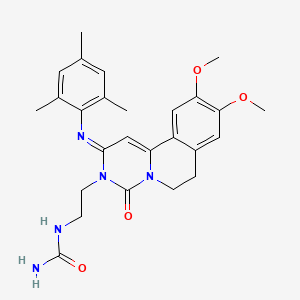

1. 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2h-pyrimidino(6,1-a)isoquinolin-4-one
2. Rpl 554
3. Rpl-554
4. Rpl554
1. Rpl-554
2. 298680-25-8
3. Rpl554
4. 1884461-72-6
5. Ensifentrine [inn]
6. Ensifentrine [usan]
7. Ls-193855
8. 3e3d8t1gix
9. Ls-193,855
10. A]isoquinolin-3(4h)-yl}ethyl)urea
11. (e)-1-(2-(2-(mesitylimino)-9,10-dimethoxy-4-oxo-6,7-dihydro-2h-pyrimido[6,1-a]isoquinolin-3(4h)-yl)ethyl)urea
12. 2-[9,10-dimethoxy-4-oxo-2-(2,4,6-trimethylphenyl)imino-6,7-dihydropyrimido[6,1-a]isoquinolin-3-yl]ethylurea
13. Trimethylphenyl)imino]-6,7-dihydro-2h-pyrimido[6,1-
14. N-(2-{(2e)-9,10-dimethoxy-4-oxo-2-[(2,4,6-
15. 2-(9,10-dimethoxy-4-oxo-2-(2,4,6-trimethylphenyl)imino-6,7-dihydropyrimido(6,1-a)isoquinolin-3-yl)ethylurea
16. N-(2-((2e)-9,10-dimetoxi-4-oxo-2-((2,4,6-trimetilfenil)imino)-6,7-dihidro-2h-pirimido(6,1-a)isoquinolein-3(4h)-il)etil)urea
17. Urea, N-(2-(6,7-dihydro-9,10-dimethoxy-4-oxo-2-((2,4,6-trimethylphenyl)imino)-2h-pyrimido(6,1-a)isoquinolin-3(4h)-yl)ethyl)-
18. Unii-3e3d8t1gix
19. Vmx-554
20. Rpl-554;ensifentrinum
21. Ensifentrine (usan/inn)
22. Ensifentrine [who-dd]
23. Schembl625876
24. Chembl4594287
25. Schembl20720900
26. Gtpl11865
27. Dtxsid00183983
28. Bcp31840
29. Ex-a6633
30. Who 10726
31. Sb19810
32. D11743
33. A937318
34. Q7277486
35. Ensifentrina; Ensifentrinum;rpl-554;rpl554;rpl 554
36. 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2h-pyrimidino(6,1-a)isoquinolin-4-one
37. 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2h-pyrimido[6,1-a]isoquinolin-4-one
38. 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2h-pyrimido[6,1-a]isoquinolin-4-one
39. 9,10-dimethoxy-2-(2.4.6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2h-pyrimido[6.1-a]isoquinolin-4-one
| Molecular Weight | 477.6 g/mol |
|---|---|
| Molecular Formula | C26H31N5O4 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 477.23760449 g/mol |
| Monoisotopic Mass | 477.23760449 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 849 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
About the Company : Established in 1984, Neuland Laboratories Limited is a publicly listed company headquartered in Hyderabad, India. The company provides solutions across the entire spectrum of the p...
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
About the Company : Since its inception in 2003, Seqens has grown to become a global leader in pharmaceutical solutions and specialty ingredients. Seqens supports its customers in developing, scaling ...
About the Company : Sintaho Pharmaceutical Co., Ltd., located in Chongqing, China, contributes to being the most dependable partner for global pharmaceutical companies. Sintaho specializes in chemosyn...
About the Company : Angene is pledged to providing quality chemicals for use in research and development and commercial manufacturing. Angenes offers over 100,000 products including lab reagents, inte...

About the Company : Chemicea Pharmaceuticals is a reputable contract research organization based in Pune, India. Specializing in chemistry-driven solutions, Chemicea offers a wide range of R&D product...

About the Company : Glixx Laboratories Inc (Glixx) is a global leading company specializing in biological research chemicals, providing services to meet the needs of biomedical research markets. With ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ohtuvayre (ensifentrine), is a first-in-class PDE3/4 inhibitor product candidate, which is being evaluated for the maintenance treatment of chronic obstructive pulmonary disease.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Ohtuvayre
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Verona Pharma Announces US FDA Approval of Ohtuvayre™ (ensifentrine)
Details : Ohtuvayre (ensifentrine), is a first-in-class PDE3/4 inhibitor product candidate, which is being evaluated for the maintenance treatment of chronic obstructive pulmonary disease.
Brand Name : Ohtuvayre
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The proceeds will support Verona’s planned commercial launch of RPL554 (ensifentrine), Verona Pharma’s first-in-class product candidate, which is under review by the US FDA for the maintenance treatment of chronic obstructive pulmonary disease.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Oaktree Capital Management, L.P
Deal Size: $650.0 million Upfront Cash: Undisclosed
Deal Type: Financing May 09, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Oaktree Capital Management, L.P
Deal Size : $650.0 million
Deal Type : Financing
Verona Pharma Announces $650 Million Strategic Financing with Oaktree and OMERS
Details : The proceeds will support Verona’s planned commercial launch of RPL554 (ensifentrine), Verona Pharma’s first-in-class product candidate, which is under review by the US FDA for the maintenance treatment of chronic obstructive pulmonary disease.
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Undisclosed
May 09, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The proceeds will support Verona’s planned commercial launch of RPL554 (ensifentrine), Verona Pharma’s first-in-class product candidate, which is under review by the US FDA for the maintenance treatment of chronic obstructive pulmonary disease.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Oxford Finance
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Financing January 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Oxford Finance
Deal Size : Undisclosed
Deal Type : Financing
Details : The proceeds will support Verona’s planned commercial launch of RPL554 (ensifentrine), Verona Pharma’s first-in-class product candidate, which is under review by the US FDA for the maintenance treatment of chronic obstructive pulmonary disease.
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Undisclosed
January 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
RPL554 (ensifentrine) is a selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 combining bronchodilator and NSAID activities in one compound. This activity has the potential to provide anti-inflammatory benefits for those with COPD.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : RPL554 (ensifentrine) is a selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 combining bronchodilator and NSAID activities in one compound. This activity has the potential to provide anti-inflammatory benefits for those with COPD.
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
RPL554 (ensifentrine) is a selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 combining bronchodilator and NSAID activities in one compound. This activity has the potential to provide anti-inflammatory benefits for those with COPD.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : RPL554 (ensifentrine) is a selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 combining bronchodilator and NSAID activities in one compound. This activity has the potential to provide anti-inflammatory benefits for those with COPD.
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
RPL554 (ensifentrine) is a selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 combining bronchodilator and NSAID activities in one compound. This activity has the potential to provide anti-inflammatory benefits for those with COPD.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Nuance Pharma Announces Dosing of First Patient in ENHANCE China Phase 3 Trial for COPD
Details : RPL554 (ensifentrine) is a selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 combining bronchodilator and NSAID activities in one compound. This activity has the potential to provide anti-inflammatory benefits for those with COPD.
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Not Applicable
April 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ensifentrine is a selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 combining bronchodilator and NSAID activities in one compound. This activity has the potential to provide anti-inflammatory benefits for those with COPD.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Ensifentrine is a selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 combining bronchodilator and NSAID activities in one compound. This activity has the potential to provide anti-inflammatory benefits for those with COPD.
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Not Applicable
April 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ensifentrine (RPL554) is an investigational, first-in-class, selective dual inhibitor of the enzymes phosphodiesterase 3 and 4 that combines bronchodilator and anti-inflammatory activities in one compound.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 20, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Ensifentrine (RPL554) is an investigational, first-in-class, selective dual inhibitor of the enzymes phosphodiesterase 3 and 4 that combines bronchodilator and anti-inflammatory activities in one compound.
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 20, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
RPL554 (ensifentrine), met the primary endpoint in ENHANCE-2 demonstrating a statistically significant and clinically meaningful improvement in lung function. In addition, ensifentrine significantly reduced the rate of COPD exacerbations in the ENHANCE-2 trial.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 19, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : RPL554 (ensifentrine), met the primary endpoint in ENHANCE-2 demonstrating a statistically significant and clinically meaningful improvement in lung function. In addition, ensifentrine significantly reduced the rate of COPD exacerbations in the ENHANCE-2...
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 19, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The non-dilutive capital provides further financial flexibility and support for commercialization activities for ensifentrine (RPL554), the first-in-class product candidate, which reported positive Phase 3 data in the ENHANCE-2 trial in chronic obstructive pulmonary disease.
Lead Product(s): Ensifentrine
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: RPL554
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Oxford Finance LLC
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Financing October 17, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ensifentrine
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Oxford Finance LLC
Deal Size : Undisclosed
Deal Type : Financing
Verona Pharma Secures Debt Financing of up to $150 Million from Oxford Finance
Details : The non-dilutive capital provides further financial flexibility and support for commercialization activities for ensifentrine (RPL554), the first-in-class product candidate, which reported positive Phase 3 data in the ENHANCE-2 trial in chronic obstructi...
Brand Name : RPL554
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 17, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : OHTUVAYRE
Dosage Form : SUSPENSION;INHALATION
Dosage Strength : 3MG/2.5ML
Approval Date : 2024-06-26
Application Number : 217389
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patents & EXCLUSIVITIES
Patent Expiration Date : 2035-09-15
US Patent Number : 10945950
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 217389
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-09-15

Patent Expiration Date : 2031-08-21
US Patent Number : 9062047
Drug Substance Claim : Y
Drug Product Claim :
Application Number : 217389
Patent Use Code : U-3962
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-08-21

Patent Expiration Date : 2035-09-15
US Patent Number : 9956171
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 217389
Patent Use Code : U-3962
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-09-15

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
