
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
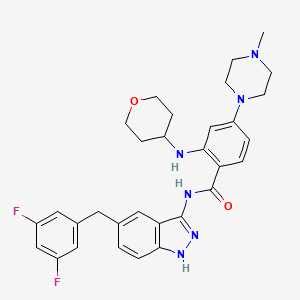

1. N-(5-(3,5-difluorobenzyl)-1h-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-(tetrahydro-2h-pyran-4-ylamino)benzamide
2. Nms-e628
3. Rozlytrek
4. Rxdx-101
1. 1108743-60-7
2. Rxdx-101
3. Nms-e628
4. Rozlytrek
5. Entrectinib (rxdx-101)
6. Entrectinib(rxdx-101)
7. L5orf0an1i
8. N-(5-(3,5-difluorobenzyl)-1h-indazol-3-yl)-4-(4-methylpiperazin-1-yl)-2-((tetrahydro-2h-pyran-4-yl)amino)benzamide
9. Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1h-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2h-pyran-4-yl)amino)-
10. N-(5-(3,5-difluorobenzyl)-1h-indazol-3-yl)-4-(4-methylpiperazin-1yl)-2-(tetrahydro-2h-pyran-4-ylamino)benzamide
11. N-[5-[(3,5-difluorophenyl)methyl]-1h-indazol-3-yl]-4-(4-methylpiperazin-1-yl)-2-(oxan-4-ylamino)benzamide
12. Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1h-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2h-pyran-4-yl)amino]-
13. N-{5-[(3,5-difluorophenyl)methyl]-1h-indazol-3-yl}-4-(4-methylpiperazin-1-yl)-2-[(oxan-4-yl)amino]benzamide
14. Entrectinib [inn]
15. Unii-l5orf0an1i
16. Entrectinib [usan:inn]
17. Rozlytrek (tn)
18. Ymx
19. Entrectinib, 95%
20. Kinome_2659
21. Entrectinib [mi]
22. Entrectinib [jan]
23. Entrectinib; Nms-e628
24. Entrectinib [usan]
25. Entrectinib [who-dd]
26. Entrectinib (jan/usan/inn)
27. Gtpl8290
28. Schembl3512601
29. Chembl1983268
30. Nms-e-628
31. Entrectinib [orange Book]
32. Nms-e628;rxdx-101
33. Dtxsid101026450
34. Hms3886h21
35. Bcp16174
36. Ex-a2261
37. Mfcd28129099
38. Nsc774769
39. Nsc800095
40. S7998
41. Zinc43204146
42. Ccg-270048
43. Db11986
44. Nsc-774769
45. Nsc-800095
46. Sb17194
47. Ncgc00484067-01
48. Ncgc00484067-02
49. Ncgc00484067-03
50. Ac-31286
51. As-75092
52. Da-47850
53. Hy-12678
54. B5859
55. Ft-0736318
56. D10926
57. A856078
58. Q25323953
59. S900006830
60. Rxdx101; Rxdx 101; Rxdx-101; Nms E628; Nms-e628;nms E628
61. N-(5-(3,5-difluorobenzyl)-1h-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-(tetrahydro-2h-pyran-4-ylamino)benzamide
62. N-(5-(3,5-difluorobenzyl)-1h-indazol-3-yl)-4-(4-methylpiperazin-1-yl)-2-(tetrahydro-2h-pyran-4-ylamino)benzamide
63. N-[5-(3,5-difluoro-benzyl)-1h-indazol-3-yl]-4-(4-methyl-piperazin-1-yl)-2-(tetrahydro-pyran-4-ylamino)-benzamide
64. N-{5-[(3,5-difluorophenyl)methyl]-3h-indazol-3-ylidene}-4-(4-methylpiperazin-1-yl)-2-[(oxan-4-yl)amino]benzamide
| Molecular Weight | 560.6 g/mol |
|---|---|
| Molecular Formula | C31H34F2N6O2 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 560.27113067 g/mol |
| Monoisotopic Mass | 560.27113067 g/mol |
| Topological Polar Surface Area | 85.5 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 847 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Entrectinib is indicated for the treatment of metastatic ROS1-positive non-small cell lung cancer in adults. Entrectinib is also indicated in adults and children over 12 years old for the treatment of NTRK gene fusion-positive solid tumors which have metastasized or for which surgical resection is likely to result in severe morbidity and for which has progressed on previous therapies or for which no comparable alternative therapies are available.
FDA Label
Rozlytrek as monotherapy is indicated for the treatment of adult and paediatric patients 12 years of age and older with solid tumours expressing a neurotrophic tyrosine receptor kinase (NTRK) gene fusion,
- who have a disease that is locally advanced, metastatic or where surgical resection is likely to result in severe morbidity, and
- who have not received a prior NTRK inhibitor
- who have no satisfactory treatment options.
Rozlytrek as monotherapy is indicated for the treatment of adult patients with ROS1 positive, advanced non small cell lung cancer (NSCLC) not previously treated with ROS1 inhibitors.
Entrectinib and its active metabolite suppress several pathways which contribute to cell survival and proliferation. This suppression shifts the balance in favor of apoptosis thereby preventing cancer cell growth and shrinking tumors.
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01EX14
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX14 - Entrectinib
Absorption
Entrectinib has a Tmax of 4-5 h after administration of a single 600 mg dose. Food does not produce a significant effect on the extent of absorption.
Route of Elimination
After a single radio-labeled dose of entrectinib, 83% of radioactivity was present in the feces and 3% in the urine. Of the dose in the feces, 36% was present as entrectinib and 22% as M5.
Volume of Distribution
Entrectinib has an apparent volume of distribution of 551 L. The active metabolite, M5, has an apparent volume of distribution of 81.1 L. Entrectinib is known to cross the blood-brain barrier.
Clearance
The apparent clearance of entrectinib is 19.6 L/h while the apparent clearance of the active metabolite M5 is 52.4 L/h.
CYP3A4 is responsible for 76% of entrectinib metabolism in humans including metabolism to the active metabolite, M5. M5 has similar pharmacological activity to entrectinib and exists at approximately 40% of the steady state concentration of the parent drug. In rats, six in vivo metabolites have been identified including N-dealkylated, N-oxide, hydroxylated, and glucuronide conjugated metabolites.
Entrectinib has a half-life of elimination of 20 h. The active metabolite, M5, has a half-life of 40 h.
Entrectinib is a tyrosine kinase inhibitor which acts on several receptors. It functions as an ATP competitor to inhibit tropomyosin receptor tyrosine kinases (TRK) TRKA, TRKB, TRKC, as well as proto-oncogene tyrosine-protein kinase ROS1 and anaplastic lymphoma kinase (ALK). TRK receptors produce cell proliferation via downstream signalling through the mitogen activated protein kinase, phosphoinositide 3-kinase, and phospholipase C-. ALK produces similar signalling with the addition of downstream JAK/STAT activation. Inhibition of these pathways suppresses cancer cell proliferation and shifts the balance in favor of apoptosis resulting in shrinking of tumor volume.
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
78
PharmaCompass offers a list of Entrectinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Entrectinib manufacturer or Entrectinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Entrectinib manufacturer or Entrectinib supplier.
PharmaCompass also assists you with knowing the Entrectinib API Price utilized in the formulation of products. Entrectinib API Price is not always fixed or binding as the Entrectinib Price is obtained through a variety of data sources. The Entrectinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Entrectinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Entrectinib, including repackagers and relabelers. The FDA regulates Entrectinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Entrectinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Entrectinib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Entrectinib supplier is an individual or a company that provides Entrectinib active pharmaceutical ingredient (API) or Entrectinib finished formulations upon request. The Entrectinib suppliers may include Entrectinib API manufacturers, exporters, distributors and traders.
click here to find a list of Entrectinib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Entrectinib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Entrectinib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Entrectinib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Entrectinib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Entrectinib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Entrectinib suppliers with NDC on PharmaCompass.
Entrectinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Entrectinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Entrectinib GMP manufacturer or Entrectinib GMP API supplier for your needs.
A Entrectinib CoA (Certificate of Analysis) is a formal document that attests to Entrectinib's compliance with Entrectinib specifications and serves as a tool for batch-level quality control.
Entrectinib CoA mostly includes findings from lab analyses of a specific batch. For each Entrectinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Entrectinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Entrectinib EP), Entrectinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Entrectinib USP).

