


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
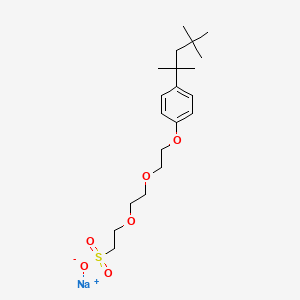

1. 2917-94-4
2. Sodium Octoxynol-2 Ethane Sulfonate
3. Entsufon Sodium [usan]
4. Triton X-200
5. L6867h5fr4
6. Entsufon Sodium (usan)
7. Sodium 2-[2-[2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethoxy]ethanesulfonate
8. Sodium 2-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethoxy)ethanesulfonate
9. Sodium;2-[2-[2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy]ethoxy]ethanesulfonate
10. Unii-l6867h5fr4
11. Einecs 220-851-3
12. Entsufon Sodium [ii]
13. Schembl767034
14. Entsufon Sodium [vandf]
15. Chembl2106682
16. Dtxsid3042398
17. Entsufon Sodium [mart.]
18. Triton X-200 [ii]
19. Entsufon Sodium [who-dd]
20. Triton X-200 Surfactant
21. 2-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethoxy)ethanesulfonic Acid, Sodium Salt
22. Sodium 2-(2-(2-(p-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethoxy)ethanesulfonate
23. Ethanesulfonic Acid, 2-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethoxy)-, Sodium Salt
24. Sodium Octoxynol-2 Ethane Sulphonate
25. Ft-0699907
26. D04009
27. Sodium Octoxynol-2 Ethane Sulfonate [inci]
28. Sodium Octoxynol-2 Ethane Sulfonate [vandf]
29. Q27282762
30. Sodium 2-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethoxy)ethanesulphonate
31. Sodium 2-(2-(2-(p-1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethoxy)ethanesulfonate
32. Ethanesulfonic Acid, 2-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethoxy)-, Sodium Salt (1:1)
| Molecular Weight | 424.5 g/mol |
|---|---|
| Molecular Formula | C20H33NaO6S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 13 |
| Exact Mass | 424.18955422 g/mol |
| Monoisotopic Mass | 424.18955422 g/mol |
| Topological Polar Surface Area | 93.3 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 502 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |


