
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
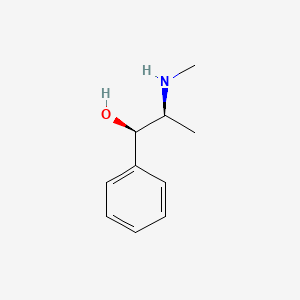

1. Ephedrine Erythro Isomer
2. Ephedrine Hydrochloride
3. Ephedrine Renaudin
4. Ephedrine Sulfate
5. Erythro Isomer Of Ephedrine
6. Hydrochloride, Ephedrine
7. Renaudin, Ephedrine
8. Sal Phedrine
9. Sal-phedrine
10. Salphedrine
11. Sulfate, Ephedrine
1. L-ephedrine
2. (-)-ephedrine
3. 299-42-3
4. Ephedrin
5. Ephedrol
6. Mandrin
7. Sanedrine
8. Fedrin
9. L(-)-ephedrine
10. 1-sedrin
11. (1r,2s)-2-(methylamino)-1-phenylpropan-1-ol
12. Ephedrital
13. Ephedrosan
14. Ephedrotal
15. Ephedsol
16. Ephendronal
17. Ephoxamin
18. Kratedyn
19. Lexofedrin
20. Manadrin
21. Vencipon
22. Efedrin
23. Zephrol
24. Nasol
25. Xenadrine
26. I-sedrin
27. Racephedrine
28. Ephedrine, Anhydrous
29. L-alpha-(1-methylaminoethyl)benzyl Alcohol
30. L-erythro-2-(methylamino)-1-phenylpropan-1-ol
31. Nci-c55652
32. Norephedrine, N-methyl-
33. Gn83c131xs
34. Biophedrin
35. Ephedremal
36. Eciphin
37. Ephedral
38. (1r,2s)-2-methylamino-1-phenylpropan-1-ol
39. Chebi:15407
40. (1r,2s)-1-phenyl-1-hydroxy-2-methylaminopropane
41. (1r,2s)-2-(methylamino)-1-phenyl-propan-1-ol
42. Nsc-8971
43. 1-phenyl-2-methylaminopropanol
44. Nsc-170951
45. L-2-methylamino-1-phenylpropanol
46. Ephedrine, L-(-)-
47. Sal-phedrine
48. Ephedrine L-form
49. Ephedrine [usan:ban]
50. 1-hydroxy-2-methylamino-1-phenylpropane
51. (-)-ephedrine Hemisulfate
52. (l)-ephedrine
53. (-)-(1r,2s)-ephedrine
54. Ephedrine (tn)
55. Ephedrine (usp)
56. Hsdb 3072
57. 1-2-methylamino-1-phenylpropanol
58. 2-methylamino-1-phenyl-1-propanol
59. Nsc 8971
60. Einecs 206-080-5
61. Nsc 170951
62. Unii-gn83c131xs
63. 1-phenyl-1-hydroxy-2-methylaminopropane
64. Alpha-hydroxy-beta-methylaminopropylbenzene
65. 1-alpha-(1-methylaminoethyl)benzyl Alcohol
66. Ai3-02761
67. Alpha-(1-(methylamino)ethyl)benzenemethanol
68. Nsc170951
69. Alpha-hydroxy-beta-methyl Amine Propylbenzene
70. (-)-alpha-(1-methylaminoethyl)benzyl Alcohol
71. 1(-)ephedrine
72. Ephedrinum, Anhydrous
73. Ephedrine [mi]
74. Ephedrine [hsdb]
75. Benzenemethanol, Alpha-(1-(methylamino)ethyl)-, (r-(r*,s*))-
76. Ephedrine [vandf]
77. Dea Code 8113
78. Ephedrine [mart.]
79. Ec 206-080-5
80. Ephedrine [who-dd]
81. Schembl4785
82. (1r,2s)-2-(methylamino)-1-phenyl-1-propanol
83. Lopac0_000501
84. Gtpl556
85. (1r, 2s)-(-)-ephedrine
86. Chembl211456
87. Benzenemethanol, Alpha-(1-(methylamino)ethyl)-, (-)-
88. Dtxsid0022985
89. Ephedrine [ep Monograph]
90. Zinc74836
91. Ephedrine [usp Monograph]
92. Benzenemethanol, Alpha-((1s)-1-(methylamino)ethyl)-, (alphar)-
93. Hy-b1195
94. Benzenemethanol, .alpha.-[(1s)-1-(methylamino)ethyl]-, (.alpha.r)-
95. Pdsp2_001327
96. Pdsp2_001330
97. (1r,2s)-(-)-ephedrine, 98%
98. Akos016011257
99. Emerphed (ephedrine Sulfate Injection)
100. Ephedrine, Anhydrous [who-ip]
101. Ccg-204592
102. Cs-4802
103. Db01364
104. Ncgc00162174-01
105. Ncgc00162174-02
106. Sbi-0051362.p003
107. Ephedrinum, Anhydrous [who-ip Latin]
108. (1r,2s)-2-methylamino-1-phenyl-1-propanol
109. C01575
110. D00124
111. (1r*,2s*)-2-methylamino-1-phenyl-1-propanol
112. Ab00375843_06
113. 299e423
114. A820118
115. Q219626
116. Sr-01000075166
117. (1r,2s)-(-)-2-methylamino-1-phenyl-1-propanol
118. J-500280
119. Sr-01000075166-1
120. (1r,2s)-(-)-2-(n-methylamino)-1-phenylpropan-1-ol
121. (1r,2s)-(-)-alpha-(1-methylaminoethyl)benzenemethanol
122. Pseudoephedrine Hydrochloride Impurity A [ep Impurity]
123. Benzenemethanol, .alpha.-(1-(methylamino)ethyl)-, (r-(r*,s*))-
124. (r-(r*,s*))-.alpha.-(1-(methylamino)ethyl)benzenemethanol [who-ip]
| Molecular Weight | 165.23 g/mol |
|---|---|
| Molecular Formula | C10H15NO |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 165.115364102 g/mol |
| Monoisotopic Mass | 165.115364102 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 121 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic alpha-Agonists; Adrenergic beta-Agonists; Adrenergic Agents; Appetite Depressants; Bronchodilator Agents; Central Nervous System Stimulants; Sympathomimetics; Vasoconstrictor Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Ephedrine hydrochloride injection is on the WHO Model list of essentials for use in spinal anesthesia during delivery, to prevent hypotension. Its use requires specialized diagnostic or monitoring facilities, and/or specialist medical care, and/or specialist training. /Ephedrine hydrochloride/
WHO Model List of Essential Medicines, 14th edition (March 2005). Available from, as of March 14, 2007: https://whqlibdoc.who.int/hq/2005/a87017_eng.pdf
Uses: Diseases of the respiratory tract with mild bronchospasms in adults and children over the age of six. /Ephedrae herba, ephedra, ma-huang/
Blumenthal M, ed.; Ephedra. In the Complete German Commission E Monographs. Therapeutic Guide to Herbal Medicines. p. 125-6. The American Botanical Council, Austin, TX (1998)
Indications and Usage: Approved by /German/ Commission E: Cough/bronchitis. Unproven Uses: Ma-huang is used for diseases of the respiratory tract with mild bronchospasms in adults and children over the age of six. Various indications include asthma, cardiovascular stimulation and as a CNS stimulant. Chinese Medicine: The drug has been used for over 4000 years for severe febrile illnesses, bronchial asthma, joint symptoms, inability to perspire, coughing with dyspnea, edema and pains in the bones. /Ma-huang (Ephedra sinica)/
PDR for Herbal Medicines, 2nd ed. Ma Huang (Ephedra sinica). p. 488-9 (2000) Medical Economics Co., Montvale, NJ
For more Therapeutic Uses (Complete) data for (L)-EPHEDRINE (9 total), please visit the HSDB record page.
Contraindications include states of anxiety and restlessness, high blood pressure, angle-closure glaucoma, cerebral perfusions, prostate adenoma with residual urine volume, pheochromocytoma and thyrotoxicosis. /Ma-huang/
PDR for Herbal Medicines, 2nd ed. Ma Huang (Ephedra sinica). p. 488-9 (2000) Medical Economics Co., Montvale, NJ
The CNS-simulating effects of ephedrine may result in nervousness, anxiety, apprehension, fear, tension, agitation, excitation, restlessness, weakness, irritability, talkativeness, or insomnia. Dizziness, lightheadedness, and vertigo may occur, especially with large doses. Tremor or tremulousness, and hyperactive reflexes have also been reported. CNS disturbances may be prevented or overcome by administration of a sedative or tranquilizer. Large parenteral doses of ephedrine may cause confusion, delirium, hallucinations, or euphoria. Some asthmatic patients receiving continuous oral administration of the drug have taken extremely high doses in attempts to overcome refractoriness. Paranoid psychosis and visual auditory hallucinations occurred in some of these patients. Withdrawal of the drug produced rapid recovery.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1352
When the drug is used topically as a nasal decongestant (the nasal solution is no longer commercially available in the US), rebound congestion and tachyphylaxis may occur within a few days. Repeated topical use of the previously available 3% solution occasionally caused local irritation. Preparations containing ephedrine in oil solutions should be avoided, especially in children, because lipid pneumonia may result. In addition, CNS stimulation or cardiovascular effects similar to those occurring after oral or parenteral use of the drug may occur.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1352
Ephedrine also may cause throbbing headache, respiratory difficulty, fever or a feeling of warmth, pallor, dryness of the nose and throat, precordial pain, sweating, mild epigastric distress, anorexia, nausea, or vomiting.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1352
For more Drug Warnings (Complete) data for (L)-EPHEDRINE (21 total), please visit the HSDB record page.
5. 5= Extremely toxic: Probable oral lethal dose (human) is 5-50 mg/kg, between 7 drops and 1 teaspoonful for 70 kg person (150 lb). The Probable lethal dose in man is 50 mg/kg.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-370
Ephedrine intravenous injections are indicated to treat hypotension under anesthesia, ephedrine injections by multiple routes are indicated to treat allergic conditions such as bronchial asthma, ephedrine nasal spray is and OTC medication used as a decongestant.
Ephedrine is a sympathomimetic amine that activates adrenergic receptors, increasing heart rate and blood pressure, and causing bronchodilation. The therapeutic window is wide as patients can be given doses of 5mg up to 50mg. Patients should be counselled regarding the pressor effects of sympathomimetic amines and the risk of tachyphylaxis.
Adrenergic Agents
Drugs that act on adrenergic receptors or affect the life cycle of adrenergic transmitters. Included here are adrenergic agonists and antagonists and agents that affect the synthesis, storage, uptake, metabolism, or release of adrenergic transmitters. (See all compounds classified as Adrenergic Agents.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
C01CA26
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C01 - Cardiac therapy
C01C - Cardiac stimulants excl. cardiac glycosides
C01CA - Adrenergic and dopaminergic agents
C01CA26 - Ephedrine
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AA - Sympathomimetics, plain
R01AA03 - Ephedrine
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AB - Sympathomimetics, combinations excl. corticosteroids
R01AB05 - Ephedrine
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03C - Adrenergics for systemic use
R03CA - Alpha- and beta-adrenoreceptor agonists
R03CA02 - Ephedrine
S - Sensory organs
S01 - Ophthalmologicals
S01F - Mydriatics and cycloplegics
S01FB - Sympathomimetics excl. antiglaucoma preparations
S01FB02 - Ephedrine
Absorption
Oral ephedrine reaches an average Cmax of 79.5ng/mL, with a Tmax of 1.81h, and a bioavailability of 88%.
Route of Elimination
Ephedrine is mainly eliminated in the urine. Approximately 60% is eliminated as the unmetabolized parent compound, 13% as benzoic acid conjugates, and 1% as 1,2-dihydroxypropylbenzene.
Volume of Distribution
Oral ephedrine has an average volume of distribution of 215.6L.
Clearance
Oral ephedrine has a clearance of 23.3L/h but there is a high degree of inter-patient variability.
Placental transfer of ephedrine occurs at 70% of the maternal blood levels. Ephedrine is also excreted in breast milk.
CANTOX: Safety Assessment and Determination of a Tolerable Upper Limit for Ephedra. Report prepared by CANTOX Health Sciences International, Ontario, for the Council for Responsible Nutrition, Washington, DC, p. 8 (2000).
Up to 95% of an oral dose may be excreted in the urine within 24 hours. The urinary excretion of ephedrine is pH-dependent due to the presence of an ionizable group in the ephedrine molecule and is increased in acidic urine. In alkaline urine, excretion is reduced to 20 to 35% of the dose.
CANTOX: Safety Assessment and Determination of a Tolerable Upper Limit for Ephedra. Report prepared by CANTOX Health Sciences International, Ontario, for the Council for Responsible Nutrition, Washington, DC, p. 7 (2000)
Ephedrine is rapidly and completely absorbed after oral, intramuscular, or subcutaneous administration.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1573
...This study aimed to develop a mechanistic model describing ephedrine, norephedrine, and caffeine pharmacokinetics and their interactions in healthy subjects. ... The treatments consisted of single-doses of pharmaceutical caffeine and ephedrine, given alone or together, and an herbal formulation containing both caffeine and ephedrine. /The authors/ used a mixed-effect statistical model and the program NONMEM to take account of intersubject variability. ... Three hundred and seventy-nine ephedrine, 352 norephedrine, 417 caffeine plasma concentrations and 40 ephedrine urine concentrations were obtained from 24 subjects. A one-compartment model with first-order absorption described the caffeine data. Caffeine clearance was 0.083 L/min (CV 38%) and decreased to 0.038 L/min in presence of oral contraceptive therapy, its volume of distribution was 38.6 L (CV 20%) and its absorption rate constant was 0.064 L/min (CV 50%). A four-compartment model described the pharmocokinetics of ephedrine and norephedrine. Ephedrine was eliminated mostly renally, with a clearance of 0.34 L/min (CV 11%), and a volume of distribution of 181 L (CV 19%). Nonlinearity in the conversion of ephedrine to norephedrine was observed. Different models showed that the simultaneous administration of caffeine, or the amount of caffeine in the absorption compartment, was associated with a slower rate of absorption of ephedrine. A 32% greater relative bioavailability of herbal compared with pharmaceutical ephedrine administration was observed. ... Concomitant ingestion of caffeine slowed the absorption rate of ephedrine, which is mainly related to the amount of the former in the absorption compartment. A saturable process appears to be involved in the metabolism of ephedrine to norephedrine.
PMID:15752380 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1884794 Csajka C et al; Br J Clin Pharmacol 59 (3): 335-45 (2005)
For more Absorption, Distribution and Excretion (Complete) data for (L)-EPHEDRINE (7 total), please visit the HSDB record page.
Ephedrine is largely unmetabolized in the body. Ephedrine can be N-demethylated to norephedrine, or demethylated and deaminized to benzoic acid conjugates and 1,2-hydroxypropylbenzene.
... After /volunteers (n=3 for each drug) consumed a single clinical dose of ephedrine (EPH), pseudoephedrine (PEPH), phenylpropanolamine (PPA), methylephedrine (MEPH) or cathine/..., urine samples were subjected to tert-butyl-methyl-ether (TBME) extraction and trifluoroacetic acid (TFAA) derivatization before gas chromatography-mass spectrometry (GC-MS) analysis. Most ephedrines were excreted unchanged in urine, including EPH (40.9%), PEPH (72.2%), and PPA (59.3%). However, only a relatively small amount of MEPH (15.5%) was excreted unchanged in urine. In addition, a trace amount of PPA (1.6%) and cathine (0.7%) was found to be the metabolites of EPH and PEPH, respectively. Urinary EPH, PEPH, and PPA reached peaks at 2-6 hr and disappeared in urine at approximately 24-48 hr post-administration. For MEPH, the peaks of excretion extended from 4 to 12 hr post-administration and were undetectable at approximately 48 hr. A single clinical dose of EPH (25 mg) may exceed threshold level (10 ug/mL) in sport drug testing if the urine samples are tested within approximately 8 hr post-administration. However, a single dose of MEPH (20 mg) never reached the threshold value (10 ug/mL).
PMID:15885945 Tseng YL et al; Forensic Sci Int 157 (2-3): 149-55 (2006)
The metabolism of ephedrine in humans, dogs and several species of rodents proceeds primarily by three reactions; aromatic hydroxylation, N-demethylation, and oxidative deamination. The extent to which ephedrine is metabolized and the major metabolites vary quantitatively between species. The extent of aromatic hydroxylation is greatest in rats, followed by rabbits, guinea pigs, and dogs, with no aromatic hydroxylation observed in humans. N-demethylation of ephedrine is greatest in rabbits followed by dogs, guinea pigs, rats, and humans. Deamination is greatest in rabbits, followed by humans and rats. Ephedrine, 8-20%, is metabolized in humans by N-demethylation to /phenylpropanolamin/ PPA. A total of 4-13% of an oral dose of ephedrine undergoes oxidative deamination yielding 1-phenylpropan-1,2-diol and further side-chain oxidation to benzoic acid and hippuric acid.
CANTOX: Safety Assessment and Determination of a Tolerable Upper Limit for Ephedra. Report prepared by CANTOX Health Sciences International, Ontario, for the Council for Responsible Nutrition, Washington, DC, p. 7 (2000)
Yields L-norephedrine and phenylglycol in rabbits. /from table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. E-2
Oral ephedrine has a plasma elimination half life of approximately 6 hours, but there is a large degree of inter-patient variability.
...serum half-life of 2-3 hr.
Thurn AL; p. 189-95 in Encyclopedia of Dietary Supplements; Coates PM et al, eds (2005)
Ephedrineis eliminated in the urine largely as unchanged drug, with a t1/2 of 3-6 hrs.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 300
Ephedrine is a direct and indirect sympathomimetic amine. Ephedrine activates adrenergic and -receptors as well as inhibiting norepinephrine reuptake, and increasing the release of norepinephrine from vesicles in nerve cells. These actions combined lead to larger quantities of norepinephrine present in the synapse, for longer periods of time, increasing stimulation of the sympathetic nervous system. Ephedrine's stimulation of -1 receptors causes constriction of veins and a rise in blood pressure, stimulation of -1 adrenergic receptors increase cardiac chronotropy and inotropy, stimulation of -2 adrenergic receptors causes bronchodilation.
Ephedrine alkaloids are members of a large family of sympathomimetic compounds that include dobutamine and amphetamine. Members of this family increase blood pressure and heart rate by binding to alpha- and beta-adrenergic receptors present in many parts of the body, including the heart and blood vessels. These compounds are called sympathomimetics because they mimic the effects of epinephrine and norepinephrine, which occur naturally in the human body. In addition to their direct pharmacological effects, many of these compounds also stimulate the release of norepinephrine from nerve endings. The release of norepinephrine further increases the sympathomimetic effects of these compounds, at least transiently.
FDA; 21 CFR Part 119 Final Rule Declaring Dietary Supplements Containing Ephedrine Alkaloids Adulterated Because They Present an Unreasonable Risk. Federal Register: February 11, 2004 (Volume 69, Number 28) Page 6787-6854
Ephedrine does not contain a catechol moiety, and it is effective after oral administration. The drug stimulates heart rate and cardiac output and variably increases peripheral resistance; as a result, ephedrine usually increases blood pressure. Stimulation of the alpha-adrenergic receptors of smooth muscle cells in the bladder base may increase the resistance to the outflow of urine. Activation of beta-adrenergic receptors in the lungs promotes bronchodilation.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 300
Ephedrine stimulates both alpha- and beta-adrenergic receptors. It is believed that beta-adrenergic effects result from stimulation of the production of cyclic adenosine 3',5'-monophosphate (AMP) by activation of the enzyme adenyl cyclase, whereas a-adrenergic effects result from inhibition of adenyl cyclase activity. In contrast to epinephrine, ephedrine also has an indirect effect by releasing norepinephrine from its storage sites. With prolonged use or if doses are given frequently, ephedrine may deplete norepinephrine stores in sympathetic nerve endings and tachyphylaxis may develop to the cardiac and pressor effects. Tachyphylaxis to the bronchial effects of the drug may also occur, but it is not the result of norepinephrine depletion.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1354

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

ABOUT THIS PAGE
44
PharmaCompass offers a list of Ephedrine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ephedrine manufacturer or Ephedrine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ephedrine manufacturer or Ephedrine supplier.
PharmaCompass also assists you with knowing the Ephedrine API Price utilized in the formulation of products. Ephedrine API Price is not always fixed or binding as the Ephedrine Price is obtained through a variety of data sources. The Ephedrine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ephedrine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ephedrine, including repackagers and relabelers. The FDA regulates Ephedrine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ephedrine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ephedrine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ephedrine supplier is an individual or a company that provides Ephedrine active pharmaceutical ingredient (API) or Ephedrine finished formulations upon request. The Ephedrine suppliers may include Ephedrine API manufacturers, exporters, distributors and traders.
click here to find a list of Ephedrine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ephedrine DMF (Drug Master File) is a document detailing the whole manufacturing process of Ephedrine active pharmaceutical ingredient (API) in detail. Different forms of Ephedrine DMFs exist exist since differing nations have different regulations, such as Ephedrine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ephedrine DMF submitted to regulatory agencies in the US is known as a USDMF. Ephedrine USDMF includes data on Ephedrine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ephedrine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ephedrine suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ephedrine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ephedrine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ephedrine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ephedrine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ephedrine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ephedrine suppliers with NDC on PharmaCompass.
Ephedrine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ephedrine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ephedrine GMP manufacturer or Ephedrine GMP API supplier for your needs.
A Ephedrine CoA (Certificate of Analysis) is a formal document that attests to Ephedrine's compliance with Ephedrine specifications and serves as a tool for batch-level quality control.
Ephedrine CoA mostly includes findings from lab analyses of a specific batch. For each Ephedrine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ephedrine may be tested according to a variety of international standards, such as European Pharmacopoeia (Ephedrine EP), Ephedrine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ephedrine USP).
