
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
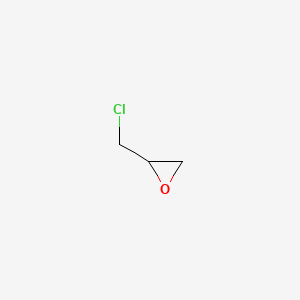

1. Epichlorhydrin
2. Epichlorohydrin, (+-)-isomer
3. Epichlorohydrin, (s)-isomer
1. 2-(chloromethyl)oxirane
2. 106-89-8
3. Epichlorhydrin
4. 1-chloro-2,3-epoxypropane
5. Oxirane, (chloromethyl)-
6. Glycidyl Chloride
7. Epichlorhydrine
8. Chloromethyloxirane
9. 1,2-epoxy-3-chloropropane
10. 2,3-epoxypropyl Chloride
11. Chloropropylene Oxide
12. 3-chloro-1,2-epoxypropane
13. Glycerol Epichlorhydrin
14. Epichloorhydrine
15. Glycerol Epichlorohydrin
16. 3-chloropropylene Oxide
17. (chloromethyl)ethylene Oxide
18. (chloromethyl)oxirane
19. Epicloridrina
20. 3-chloro-1,2-propylene Oxide
21. Epichlorohydryna
22. Epichlorophydrin
23. Alpha-epichlorohydrin
24. Epi-chlorohydrin
25. 3-chloropropene-1,2-oxide
26. Skekhg
27. (+/-)-epichlorohydrin
28. Oxirane, 2-(chloromethyl)
29. 2-chloromethyl-oxirane
30. Epoxypropyl Chloride
31. Gamma-chloropropylene Oxide
32. Propane, 1-chloro-2,3-epoxy-
33. 1-chlor-2,3-epoxy-propan
34. 1-cloro-2,3-epossipropano
35. 1-chloor-2,3-epoxy-propaan
36. Rcra Waste Number U041
37. (+/-)-2-(chloromethyl)oxirane
38. Allyl Chloride Oxide
39. (rs)-3-chloro-1,2-epoxypropane
40. Dl-a-epichlorohydrin
41. Epoxy-3-chloropropane
42. (chloromethyl)-oxirane
43. Chloropropylene
44. .alpha.-epichlorohydrin
45. Epichlorohydrine
46. Nsc 6747
47. Alyl Chloride Oxide
48. Chloropropyl Epoxide
49. .gamma.-chloropropylene Oxide
50. (dl)-.alpha.-epichlorohydrin
51. Oxirane,(chloromethyl)-
52. Chloro-1,2-epoxypropane
53. Chloro-2,3-epoxypropane
54. Chloropropene-1,2-oxide
55. 3-chloro-propylene Oxide
56. 2-(chloromethyl)-oxirane
57. Chloro-1,2-propylene Oxide
58. 1-chloro-2,3-epoxy-propane
59. 1-chloro-2,3-epoxy Propone
60. Chebi:37144
61. 08oor508c0
62. Nsc-6747
63. Mfcd00005132
64. Oxirane, (chloromethyl)-, Homopolymer
65. Ncgc00091792-01
66. Dsstox_cid_566
67. Epichlorohydrin, >=99%
68. Dsstox_rid_75662
69. Dsstox_gsid_20566
70. 13403-37-7
71. 9009-12-5
72. Ech
73. Caswell No. 424
74. Epicloridrina [italian]
75. Epichloorhydrine [dutch]
76. Epichlorhydrine [french]
77. Epichlorohydryna [polish]
78. Cas-106-89-8
79. Hsdb 39
80. Ccris 277
81. 3-chloropropyl Epoxide
82. Nci-c07001
83. 5-17-01-00020 (beilstein Handbook Reference)
84. Einecs 203-439-8
85. Un2023
86. 1-chlor-2,3-epoxy-propan [german]
87. Rcra Waste No. U041
88. 1-chloor-2,3-epoxy-propaan [dutch]
89. 1-cloro-2,3-epossipropano [italian]
90. Epa Pesticide Chemical Code 097201
91. Brn 0079785
92. Epichiorohydrin
93. (chloromethyl) Ethylene Oxide
94. Unii-08oor508c0
95. Ai3-03545
96. Epi-chlorohydrine
97. A-epichlorohydrin
98. Epichloro Hydrine
99. (rs)-epichlorohydrin
100. (+) Epichlorohydrin
101. (-) Epichlorohydrin
102. 2-chloromethyloxirane
103. (?)-epichlorohydrin
104. Chloromethyl) Oxirane
105. Poly(epichlorohydrin)
106. (rac)-epichlorohydrin
107. Racemic Epichlorohydrin
108. Beta-epoxypropylchloride
109. Racemic Epichlorohydrine
110. Epichlorohydrin, 99%
111. Poly(chloromethyloxirane)
112. 2-(chloromethyl) Oxirane
113. Epichlorohydrin Homopolymer
114. Bmse000722
115. Ec 203-439-8
116. Wln: T3otj B1g
117. (dl)-alpha-epichlorohydrin
118. (rs)-(chloromethyl)oxirane
119. Epichlorohydrin [mi]
120. Epichlorohydrin Reagent Grade
121. Oxirane, 2-(chloromethyl)-
122. Epichlorohydrin [hsdb]
123. Epichlorohydrin [iarc]
124. Epichlorohydrin [inci]
125. E181 [russian Epoxy Resin]
126. E 181 [russian Epoxy Resin]
127. Epichlorohydrin [mart.]
128. Chembl1421613
129. Dtxsid1020566
130. Nsc6747
131. (chloromethyl)oxirane, Homopolymer
132. Amy40813
133. Tox21_111167
134. Tox21_200276
135. Stl163564
136. (+/-)-1-chloro-2,3-epoxypropane
137. Akos000118974
138. Akos016039400
139. Epichlorohydrin [un2023] [poison]
140. Sb11597
141. Sb11598
142. Un 2023
143. Ncgc00091792-02
144. Ncgc00091792-03
145. Ncgc00257830-01
146. Bp-31004
147. Bp-31046
148. (+/-)-epichlorohydrin, Analytical Standard
149. Db-018066
150. Epichlorhydrin 1000 Microg/ml In Methanol
151. Epichlorhydrin 100 Microg/ml In Cyclohexane
152. Ft-0605064
153. Ft-0605270
154. Ft-0625672
155. Ft-0667883
156. Epichlorhydrin 100 Microg/ml In Acetonitrile
157. (+/-)-epichlorohydrin, Purum, >=99% (gc)
158. Q423083
159. (+/-)-epichlorohydrin, Puriss., >=99.5% (gc)
160. Q-201062
161. F0001-0128
162. Z955123632
163. (+/-)-2-(chloromethyl)oxirane, 1-chloro-2,3-epoxypropane
| Molecular Weight | 92.52 g/mol |
|---|---|
| Molecular Formula | C3H5ClO |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 92.0028925 g/mol |
| Monoisotopic Mass | 92.0028925 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 37.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
WITH EXCEPTION OF PANCREAS, A CORRELATION OBSERVED BETWEEN TISSUE DISTRIBUTION & TARGET ORGAN TOXICITY. EXCRETION MAINLY IN URINE. 21 & 18% OF DOSE EXCRETED AS CO2 IN MALE & FEMALE RATS. PEAK TISSUE LEVELS IN FEMALES LOWER.
WEIGEL WW ET AL, RES COMMUN CHEM PATHOL PHARMACOL 20 (2): 275 (1978)
/EPICHLOROHYDRIN MAY BE/ ABSORBED THROUGH THE SKIN.
Mackison, F. W., R. S. Stricoff, and L. J. Partridge, Jr. (eds.). NIOSH/OSHA - Occupational Health Guidelines for Chemical Hazards. DHHS(NIOSH) Publication No. 81-123 (3 VOLS). Washington, DC: U.S. Government Printing Office, Jan. 1981.
The upper respiratory tracts of male Fischer F344 rats were surgically isolated and connected to a specially designed flow system. The tracheal connection of the upper respiratory tract and the lower respiratory tract was interrupted. The upper respiratory tract was exposed to propylene-glycol- monomethyl ether, propylene glycol monomethyl ether acetate, epichlorohydrin, cmpd which include vapors while the rat spontaneously breathed from a stream of air. Intact rats were exposed nose only to the same compound and the percentages of vapor absorbed were determined for comparison purposes. Attempts were made to correlate the results with the water solubility of the compounds. The data were compared to predictions of two compartment mathematical models. More than 50 to 70% of the epichlorohydrin, vapors passing through the isolated upper respiratory tacts were absorbed. With the exception of styrene and methylene chloride, the percentage of vapors absorbed by the upper respiratory tract approximated that observed in the lower respiratory tract and nose only exposed animals. There was no correlation between absorption in the URT and water solubility. The mathematical models generally predicted the absorption of vapors by the lower respiratory tract and intact animals accurately. The models seriously underestimated absorption of epichlorohydrin, by the upper respiratory tract. /Results indicate/ that blood air partitioning can account for absorption of chemicals by the upper respiratory tract, but only if other metabolic and physiological parameters are considered.
Stott Wt et al, eds; Conf. on Toxicology of Nasal Passages: Chemical Industry Institute Toxicology Series p.191-210 (1986)
The highest tissue concentrations in rodents were found in the nose after inhalation, and in the stomach after ingestion. In rats, regardless of the route of exposure, most absorbed epichlorohydrin is metabolized rapidly, part being excreted as carbon dioxide via the lungs and part as water-soluble compounds via the urine.
IPCS; Health and safety guide No. 8 on Epichlorohydrin (1987). Available from, as of March 9, 2009: https://www.inchem.org/documents/hsg/hsg/hsg008.htm
For more Absorption, Distribution and Excretion (Complete) data for EPICHLOROHYDRIN (7 total), please visit the HSDB record page.
... WISTAR RATS DOSED ORALLY OR IP WITH ... EPICHLOROHYDRIN YIELD ... URINARY METABOLITES ... 2,3-DIHYDROXYPROPYL-S-CYSTEINE & ITS N-ACETATE. SINCE EPICHLOROHYDRIN IS A STRONG ELECTROPHILE THAT IS CAPABLE OF REACTING WITH CELLULAR NUCLEOPHILES, IT IS PROBABLE THAT SOME EPICHLOROHYDRIN METABOLITES ARE ALSO COVALENTLY BOUND TO VARIOUS TISSUE MACROMOLECULES.
National Research Council. Drinking Water and Health. Volume 3. Washington, DC: National Academy Press, 1980., p. 113
Rats given oral (14)C labeled epichlorhydrin and sacrificed after 3 days: 38% exhaled as carbon dioxide; 50% excreted as metabolites in urine; 3% excreted in the feces; and the remainder was found in the tissues - liver, kidney, and forestomach.
Gingell R et al; Drug Metb Dispos 13 (3): 333-41 (1985)
The major urinary metabolite of (14)C-epichlorohydrin, after oral administration to rats, was identified previously to be N-acetyl-S-(3-chloro-2-hydroxypropyl)-L-cysteine at 36% of the administered dose. In a similar study reported here, 1,2-dibromo-3- chloropropane was metabolized to at least 20 radioactive urinary metabolites. N-acetyl-S-(3-chloro-2-hydroxypropyl)-L-cysteine was only a minor metabolite (4%) of 1,2-dibromo-3-chloropropane. Epichlorohydrin was metabolized in vitro by rat liver microsomes to alpha-chlorohydrin, but 1,2-dibromo-3-chloroproane was not metabolized to epichlorohydrin or alpha-chlorohydrin under similar conditions. Covalent binding of radioactivity to liver microsomal proteins occurred for both substrates, but was less for (14)C-epichlorohydrin than for (14)C-1,2-dibromo-3-chloropropane. Addition of 3,3,3-trichloropropylene oxide, an inhibitor of epoxide hydrolase, increased the extent of protein binding of epichlorohydrin, but decreased the amojnt of (14)C-1,2-dibromo-3-chloropropane which was bound. The data indicate the epichlorohydrin is not a significant in vivo nor in vitro metabolite of 1,2-dibromo-3-chloropropane in the rat, and is unlikely to be responsible for the toxicity of 1,2-dibromo-3-chloropropane.
PMID:3564537 Gingell R et al; Xenobiotica 17 (2): 229-40 (1987)
In in vitro studies, it was shown that epichlorohydrin was hydrolyzed into 3-chloro-1,2-propanediol by the microsomal epoxide hydrolase(s) (EC 3.3.2.3) of mouse liver in the absence of NADPH, the roles of protein or glutathione in this detoxification being insignificant.
WHO; Environmental Health Criteria Document No. 33: Epichlorohydrin (106-89-8). Available from, as of March 9, 2009: https://www.inchem.org/documents/ehc/ehc/ehc33.htm
For more Metabolism/Metabolites (Complete) data for EPICHLOROHYDRIN (7 total), please visit the HSDB record page.
No reports found; [TDR, p. 619]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 619
... Male mice were given a single ip injection of epichlorohydrin dissolved in corn oil. Groups of ten mice were killed by decapitation at 1, 3, 5, 7, 10, 15, 20 and 30 minutes after injection and blood samples were collected. The in vivo half-life of epichlorohydrin is extremely short, being only just detectable after 15 minutes.
USEPA; Health Assessment Document: Epichlorohydrin p.4-2 (1983) EPA 600/8-83-032A
Half-life values of ECH and its metabolite, alpha-chloro-hydrin, in the blood of Swiss-Albino mice, which has been administered 200 mg/kg bw doses of the substances (oral or ip) were found to be 3.6-5.3 and 65 minutes.
European Commission, ESIS; IUCLID Dataset, 1-chloro-2,3-epoxypropane (106-89-8) p 75 (2000 CD-ROM edition). Available from, as of March, 4 2009: https://esis.jrc.ec.europa.eu/
The two reactive electrophilic sites, the C-1 carbon in the epoxide ring and C-3, the chlorine-bearing carbon, behave as alkylating agents and can react nonenzymatically with glutathione or protein sulfhydryl groups.
USEPA; Health Assessment Document: Epichlorohydrin p.4-6 (1983) EPA 600/8-83-032A
3-Chloroglycerophosphate inhibited rat sperm enzyme activities (glyceraldehyde-3-phosphate dehydrogenase and triosephosphate isomerase) and hence glycolysis. Only the S(-) isomer and not the R(+) isomer of 3-chloro-1,2-propanediol produced antifertility or antiglycolytic effects. Since epichlorohydrin has not been shown to have enzyme inhibitory effects, it may be that it is metabolized in vivo to S(-) alpha-chlorohydrin phosphate, to exert its antifertility effect.
USEPA; Health Assessment Document: Epichlorohydrin p.4-8 (1983) EPA 600/8-83-032A
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
77
PharmaCompass offers a list of Epichlorohydrin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Epichlorohydrin manufacturer or Epichlorohydrin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Epichlorohydrin manufacturer or Epichlorohydrin supplier.
PharmaCompass also assists you with knowing the Epichlorohydrin API Price utilized in the formulation of products. Epichlorohydrin API Price is not always fixed or binding as the Epichlorohydrin Price is obtained through a variety of data sources. The Epichlorohydrin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Epichlorohydrin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Epichlorohydrin, including repackagers and relabelers. The FDA regulates Epichlorohydrin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Epichlorohydrin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Epichlorohydrin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Epichlorohydrin supplier is an individual or a company that provides Epichlorohydrin active pharmaceutical ingredient (API) or Epichlorohydrin finished formulations upon request. The Epichlorohydrin suppliers may include Epichlorohydrin API manufacturers, exporters, distributors and traders.
click here to find a list of Epichlorohydrin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Epichlorohydrin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Epichlorohydrin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Epichlorohydrin GMP manufacturer or Epichlorohydrin GMP API supplier for your needs.
A Epichlorohydrin CoA (Certificate of Analysis) is a formal document that attests to Epichlorohydrin's compliance with Epichlorohydrin specifications and serves as a tool for batch-level quality control.
Epichlorohydrin CoA mostly includes findings from lab analyses of a specific batch. For each Epichlorohydrin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Epichlorohydrin may be tested according to a variety of international standards, such as European Pharmacopoeia (Epichlorohydrin EP), Epichlorohydrin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Epichlorohydrin USP).
