
Synopsis
Synopsis
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
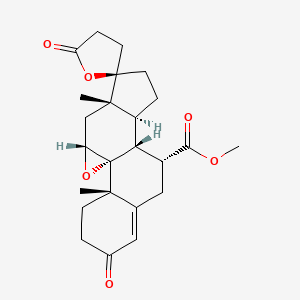

1. 9,11-epoxy-7-(methoxycarbonyl)-3-oxo-17-pregn-4-ene-21,17-carbolactone
2. Eplerenon
3. Inspra
1. Epoxymexrenone
2. Inspra
3. 107724-20-9
4. Sc-66110
5. Selara
6. Epleremone
7. Cgp 30083
8. Cgp-30083
9. Chembl1095097
10. Chebi:31547
11. Sc-6611o
12. 6995v82d0b
13. Ncgc00159559-02
14. Dsstox_cid_26094
15. Dsstox_rid_81333
16. Dsstox_gsid_46094
17. (+)-eplerenone
18. Eplerenone [usan]
19. Methyl (1'r,2r,2's,9'r,10'r,11's,15's,17'r)-2',15'-dimethyl-5,5'-dioxo-18'-oxaspiro[oxolane-2,14'-pentacyclo[8.8.0.0^{1,17}.0^{2,7}.0^{11,15}]octadecan]-6'-ene-9'-carboxylate
20. Methyl (1'r,2s,2's,9'r,10'r,11's,15's,17'r)-2',15'-dimethyl-5,5'-dioxo-18'-oxaspiro[oxolane-2,14'-pentacyclo[8.8.0.0^{1,17}.0^{2,7}.0^{11,15}]octadecan]-6'-ene-9'-carboxylate
21. Inspra (tn)
22. Cas-107724-20-9
23. Hsdb 7522
24. Eplerenone [usan:inn:ban]
25. Unii-6995v82d0b
26. Eplerenone- Bio-x
27. Sc 6110
28. Eplerenone [mi]
29. Eplerenone [inn]
30. Eplerenone [jan]
31. Eplerenone [hsdb]
32. Eplerenone [vandf]
33. Eplerenone [mart.]
34. Eplerenone [usp-rs]
35. Eplerenone [who-dd]
36. Schembl21515
37. 9,11alpha-epoxy-17-hydroxy-3-oxo-17alpha-pregn-4-ene-7alpha,21-dicarboxylic Acid, Gamma-lactone, Methyl Ester
38. Gtpl2876
39. Dtxsid2046094
40. Eplerenone (jp17/usan/inn)
41. Eplerenone [orange Book]
42. Eplerenone, >=98% (hplc)
43. Eplerenone [ep Monograph]
44. Hms3413k08
45. Hms3677k08
46. Hy-b0251
47. Zinc3985982
48. Tox21_111746
49. Bdbm50318300
50. Mfcd05662207
51. S1707
52. Akos015962307
53. Tox21_111746_1
54. Ac-4213
55. Ccg-268820
56. Db00700
57. Ks-1406
58. 7alpha-methoxycarbonyl-3-oxo-9,11alpha-epoxy-17alpha-pregn-4-ene-21,17-carbolactone
59. Ncgc00159559-03
60. Be164415
61. Pregn-4-ene-7,21-dicarboxylic Acid, 9,11-epoxy-17-hydroxy-3-oxo-, Gamma-lactone, Methyl Ester, (7alpha,11alpha,17alpha)-
62. Spiro(cyclopenta(7,8)phenanthro(4b,5-b)oxirene-7(3h),2'(3'h)-furan)-10-carboxylic Acid, 2,4,4',4a,5',5a,6,6a,8,9,9a,9b,10,11-tetradecahydro-4a,6a-dimethyl-2,5'-dioxo-, Methyl Ester, (4as,4br,5ar,6as,7 R,9as,9br,10r)-
63. E0905
64. D01115
65. Ab01274707-01
66. Ab01274707_02
67. A895400
68. Q423804
69. Sr-01000942233
70. Sr-01000942233-1
71. Methyl Dimethyl-5'-dioxo-spiro[[?]-[?],2'-tetrahydrofuran]carboxylate
72. (7?,11?,17?)-9,11-epoxy-17-hydroxy-3-oxo-pregn-4-ene-7,21-dicarboxylic Acid ?-lactone Methyl Ester
73. 9,11.alpha.-epoxy-17-hydroxy-3-oxo-17.alpha.-pregn-4-ene-7.alpha.,21-dicarboxylic Acid, .gamma.-lactone, Methyl Ester
74. 9,11alpha-epoxy-17-hydroxy-3-oxo-17alpha-pregn-4-ene-7alpha,21-dicarboxylic Acid Gamma-lactone Methyl Ester
75. Pregn-4-ene-7,21-dicarboxylic Acid, 9,11-epoxy-17-hydroxy-3-oxo-, G-lactone, Methyl Ester, (7.alpha.,11.alpha.,17.alpha)-
76. Ynu
| Molecular Weight | 414.5 g/mol |
|---|---|
| Molecular Formula | C24H30O6 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 414.20423867 g/mol |
| Monoisotopic Mass | 414.20423867 g/mol |
| Topological Polar Surface Area | 82.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 907 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Eplerenone |
| PubMed Health | Eplerenone (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Eplerenone tablets contain eplerenone, a blocker of aldosterone binding at the mineralocorticoid receptor. Eplerenone is chemically described as Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, -lactone, methyl ester, (7,11,17... |
| Active Ingredient | Eplerenone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Apotex; Sandoz |
| 2 of 4 | |
|---|---|
| Drug Name | Inspra |
| PubMed Health | Eplerenone (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | INSPRA contains eplerenone, a blocker of aldosterone binding at the mineralocorticoid receptor. Eplerenone is chemically described as Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, -lactone, methyl ester, (7,11,17)-. Its em... |
| Active Ingredient | Eplerenone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 3 of 4 | |
|---|---|
| Drug Name | Eplerenone |
| PubMed Health | Eplerenone (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Eplerenone tablets contain eplerenone, a blocker of aldosterone binding at the mineralocorticoid receptor. Eplerenone is chemically described as Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, -lactone, methyl ester, (7,11,17... |
| Active Ingredient | Eplerenone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Apotex; Sandoz |
| 4 of 4 | |
|---|---|
| Drug Name | Inspra |
| PubMed Health | Eplerenone (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | INSPRA contains eplerenone, a blocker of aldosterone binding at the mineralocorticoid receptor. Eplerenone is chemically described as Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, -lactone, methyl ester, (7,11,17)-. Its em... |
| Active Ingredient | Eplerenone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg |
| Market Status | Prescription |
| Company | Gd Searle |
Eplerenone is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive drugs. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1245
Inspra is indicated to improve survival of stable patients with left ventricular systolic dysfunction (ejection fraction less than or equal to 40%) and clinical evidence of congestive heart failure after an acute myocardial infarction.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2537
... Eplerenone should replace spironolactone as a natriuretic and antikaliuretic in heart failure and as add-on treatment in severe systolic cardiac insufficiency, and it is indicated after an acute myocardial infarction complicated by left ventricular dysfunction and heart failure. The finding that hypertension control with diuretic-based pharmacotherapy results in better prevention of heart failure than pressure reduction with other drugs makes it pertinent to investigate whether diuretics in general, and eplerenone in particular, should constitute part of the initial pharmacotherapy for heart failure when there is no overt fluid retention and independent of the etiology. ...
PMID:15733814 Reyes AJ et al; Eur J Intern Med 16 (1): 3-11 (2005)
FDA Pregnancy Risk Category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1246
... When used for hypertension, the drug is contraindicated in patients with type 2 diabetes mellitus with microalbuminuria, serum creatinine concentrations exceeding 2 or 1.8 mg/dL in males or females, respectively, creatinine clearance less than 50 mL/minute, ... .
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1979
The most serious risk associated with eplerenone therapy is hyperkalemia (serum potassium greater than 5.5 mEq/L), which may cause serious, sometimes fatal, cardiac arrhythmias. Patients with impaired renal function or diabetes mellitus and patients receiving concurrent agents affecting the renin-angiotensin-aldosterone system (e.g., angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists) are at an increased risk for developing hyperkalemia. Eplerenone should be used with caution in patients with congestive heart failure following an acute myocardial infarction, who have renal impairment (i.e., serum creatinine concentrations exceeding 2 or 1.8 mg/dL in males or females, respectively, or creatinine clearance of 50 mL/minute or less) or those with diabetes mellitus (including those with proteinuria). Serum potassium concentrations should be monitored periodically in patients receiving eplerenone. Dosage reduction has been shown to decrease serum potassium concentrations.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1979
Adverse effects reported in 1% or more of patients receiving eplerenone for the management of hypertension are dizziness, fatigue, flu-like symptoms, cough, diarrhea, abdominal pain, hyperkalemia, decreased serum sodium concentrations, abnormal vaginal bleeding, gynecomastia, hypercholesterolemia, hypertriglyceridemia, mastodynia, or albuminuria.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1979
For more Drug Warnings (Complete) data for EPLERENONE (12 total), please visit the HSDB record page.
For improvement of survival of stable patients with left ventricular systolic dysfunction (ejection fraction <40%) and clinical evidence of congestive heart failure after an acute myocardial infarction.
FDA Label
Eplerenone, an aldosterone receptor antagonist similar to spironolactone, has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulating levels do not overcome the effects of eplerenone. Eplerenone selectively binds to recombinant human mineralocorticoid receptors relative to its binding to recombinant human glucocorticoid, progesterone and androgen receptors.
Mineralocorticoid Receptor Antagonists
Drugs that bind to and block the activation of MINERALOCORTICOID RECEPTORS by MINERALOCORTICOIDS such as ALDOSTERONE. (See all compounds classified as Mineralocorticoid Receptor Antagonists.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C03DA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C03 - Diuretics
C03D - Aldosterone antagonists and other potassium-sparing agents
C03DA - Aldosterone antagonists
C03DA04 - Eplerenone
Absorption
The absolute bioavailability of eplerenone is unknown.
Volume of Distribution
43 to 90 L
Clearance
Apparent plasma cl=10 L/hr
Apparent plasma clearance: approximately 10 L/hr. Less than 5% is recovered as unchanged drug in the urine and feces. Renal: 67%. Fecal: 32%.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1245
Mean peak plasma concentrations of eplerenone are reached approximately 1.5 hours following oral administration. The absolute bioavailability of eplerenone is unknown. Both peak plasma levels (Cmax) and area under the curve (AUC) are dose proportional for doses of 25 to 100 mg and less than proportional at doses above 100 mg. The plasma protein binding of eplerenone is about 50% and it is primarily bound to alpha 1-acid glycoproteins. The apparent volume of distribution at steady state ranged from 43 to 90 L. Eplerenone does not preferentially bind to red blood cells.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2536
Eplerenone is distributed into milk in rats; ... .
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1979
... Preclinical data show that eplerenone and/or metabolites are present in rat breast milk (0.85:1 [milk:plasma] AUC ratio) obtained after a single oral dose. Peak concentrations in plasma and milk were obtained from 0.5 to 1 hour after dosing.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2538
Eplerenone is metabolized primarily by CYP3A4, however, no active metabolites have been identified in human plasma.
Eplerenone metabolism is primarily mediated via CYP3A4. No active metabolites have been identified in human plasma.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1245
Eplerenone has known human metabolites that include 21-hydroxyeplerenone and 6beta-hydroxyeplerenone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
4-6 hours
Elimination: 4 to 6 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1245
Eplerenone binds to the mineralocorticoid receptor and thereby blocks the binding of aldosterone (component of the renin-angiotensin-aldosterone-system, or RAAS). Aldosterone synthesis, which occurs primarily in the adrenal gland, is modulated by multiple factors, including angiotensin II and non-RAAS mediators such as adrenocorticotropic hormone (ACTH) and potassium. Aldosterone binds to mineralocorticoid receptors in both epithelial (e.g., kidney) and nonepithelial (e.g., heart, blood vessels, and brain) tissues and increases blood pressure through induction of sodium reabsorption and possibly other mechanisms.
Eplerenone has relative selectivity in binding to recombinant human mineralocorticoid receptors compared to its binding to recombinant human glucocorticoid, progesterone, and androgen receptors.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1245
Eplerenone has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with the inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulation levels do not overcome the effect of eplerenone on blood pressure.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1245
Eplerenone binds to the mineralocorticoid receptor and blocks the binding of aldosterone, a component of the renin-angiotensin-aldosterone-system (RAAS). Aldosterone synthesis, which occurs primarily in the adrenal gland, is modulated by multiple factors, including angiotensin II and non-RAAS mediators such as adrenocorticotropic hormone (ACTH) and potassium. Aldosterone binds to mineralocorticoid receptors in both epithelial (e.g., kidney) and nonepithelial (e.g., heart, blood vessels, brain) tissues and increases blood pressure through induction of sodium resorption and possibly other mechanisms.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1245

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
80
PharmaCompass offers a list of Eplerenone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Eplerenone manufacturer or Eplerenone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Eplerenone manufacturer or Eplerenone supplier.
PharmaCompass also assists you with knowing the Eplerenone API Price utilized in the formulation of products. Eplerenone API Price is not always fixed or binding as the Eplerenone Price is obtained through a variety of data sources. The Eplerenone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Eplerenone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Eplerenone, including repackagers and relabelers. The FDA regulates Eplerenone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Eplerenone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Eplerenone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Eplerenone supplier is an individual or a company that provides Eplerenone active pharmaceutical ingredient (API) or Eplerenone finished formulations upon request. The Eplerenone suppliers may include Eplerenone API manufacturers, exporters, distributors and traders.
click here to find a list of Eplerenone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Eplerenone DMF (Drug Master File) is a document detailing the whole manufacturing process of Eplerenone active pharmaceutical ingredient (API) in detail. Different forms of Eplerenone DMFs exist exist since differing nations have different regulations, such as Eplerenone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Eplerenone DMF submitted to regulatory agencies in the US is known as a USDMF. Eplerenone USDMF includes data on Eplerenone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Eplerenone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Eplerenone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Eplerenone Drug Master File in Japan (Eplerenone JDMF) empowers Eplerenone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Eplerenone JDMF during the approval evaluation for pharmaceutical products. At the time of Eplerenone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Eplerenone suppliers with JDMF on PharmaCompass.
A Eplerenone CEP of the European Pharmacopoeia monograph is often referred to as a Eplerenone Certificate of Suitability (COS). The purpose of a Eplerenone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Eplerenone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Eplerenone to their clients by showing that a Eplerenone CEP has been issued for it. The manufacturer submits a Eplerenone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Eplerenone CEP holder for the record. Additionally, the data presented in the Eplerenone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Eplerenone DMF.
A Eplerenone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Eplerenone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Eplerenone suppliers with CEP (COS) on PharmaCompass.
A Eplerenone written confirmation (Eplerenone WC) is an official document issued by a regulatory agency to a Eplerenone manufacturer, verifying that the manufacturing facility of a Eplerenone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Eplerenone APIs or Eplerenone finished pharmaceutical products to another nation, regulatory agencies frequently require a Eplerenone WC (written confirmation) as part of the regulatory process.
click here to find a list of Eplerenone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Eplerenone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Eplerenone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Eplerenone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Eplerenone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Eplerenone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Eplerenone suppliers with NDC on PharmaCompass.
Eplerenone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Eplerenone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Eplerenone GMP manufacturer or Eplerenone GMP API supplier for your needs.
A Eplerenone CoA (Certificate of Analysis) is a formal document that attests to Eplerenone's compliance with Eplerenone specifications and serves as a tool for batch-level quality control.
Eplerenone CoA mostly includes findings from lab analyses of a specific batch. For each Eplerenone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Eplerenone may be tested according to a variety of international standards, such as European Pharmacopoeia (Eplerenone EP), Eplerenone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Eplerenone USP).
