
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
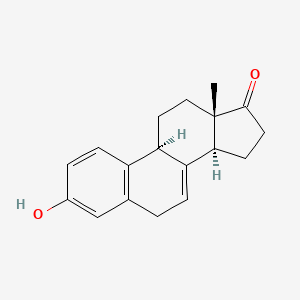

1. 474-86-2
2. 7-dehydroestrone
3. Dihydroequilenin
4. 3-hydroxyestra-1,3,5(10),7-tetraen-17-one
5. Estra-1,3,5(10),7-tetraen-17-one, 3-hydroxy-
6. 1,3,5,7-estratetraen-3-ol-17-one
7. Equilin [usp]
8. (9s,13s,14s)-3-hydroxy-13-methyl-9,11,12,14,15,16-hexahydro-6h-cyclopenta[a]phenanthren-17-one
9. Nsc-10971
10. Mls000028624
11. Chebi:42309
12. 08o86ex0j4
13. Equilin (usp)
14. Smr000058656
15. 3-hydroxy-estra-1,3,5(10),7tetraen-17-one
16. Unii-08o86ex0j4
17. Ccris 9074
18. 3-hydroxyoestra-1,3,5(10),7-tetraen-17-one
19. Cas-474-86-2
20. Prestwick_219
21. Einecs 207-488-6
22. Nsc 10971
23. Brn 2624302
24. Opera_id_780
25. Equilin [mi]
26. Equilin [mart.]
27. Prestwick0_000850
28. Prestwick1_000850
29. Prestwick2_000850
30. Prestwick3_000850
31. Equilin [usp-rs]
32. Equilin [who-dd]
33. Dsstox_cid_27433
34. Dsstox_rid_82343
35. Dsstox_gsid_47433
36. Bspbio_000839
37. 4-08-00-01366 (beilstein Handbook Reference)
38. Mls001148117
39. Schembl124758
40. Spbio_002760
41. Bpbio1_000923
42. Chembl323533
43. Equilin [usp Monograph]
44. Dtxsid7047433
45. Hms1570j21
46. Hms2097j21
47. Hms2233a16
48. Hms3714j21
49. Hy-b1176
50. Nsc10971
51. Tox21_302641
52. 1,5,7-estratetraen-3-ol-17-one
53. Bdbm50423544
54. Lmst02010026
55. Akos024285096
56. Zinc100031739
57. Ccg-220850
58. Cs-4786
59. Db02187
60. Ncgc00179406-01
61. Ncgc00256728-01
62. Equilin, Vetranal(tm), Analytical Standard
63. 3-hydroxyestra-1,5(10),7-tetraen-17-one
64. Wln: L E5 B666 Fv Juttt&j E1 Oq
65. D04041
66. S00287
67. 3-hydroxy-estra-1,3,5(10), 7-tetraen-17-one
68. 3-hydroxy-estra-1,3,5(10),7-tetraen-17-one
69. Estra-1,5(10),7-tetraen-17-one, 3-hydroxy-
70. Sr-01000721841
71. Q5384492
72. Sr-01000721841-2
73. Brd-k04046242-001-03-6
74. Equilin, European Pharmacopoeia (ep) Reference Standard
75. Equilin, United States Pharmacopeia (usp) Reference Standard
76. Equilin Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
77. (9s,13s,14s)-3-hydroxy-13-methyl-9,11,12,13,15,16-hexahydro-6h-cyclopenta[a]phenanthren-17(14h)-one
78. (9s,13s,14s)-3-hydroxy-13-methyl-9,11,12,14,15,16-hexahydro- 6h-cyclopenta[a]phenanthren-17-one
79. Equilin; 7-dehydroestrone; 3-hydroxy-1,3,5(10),7-estratetraen-17-one; 1,3,5(10),7-estratetraen-3-ol-17-one
| Molecular Weight | 268.3 g/mol |
|---|---|
| Molecular Formula | C18H20O2 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 268.146329876 g/mol |
| Monoisotopic Mass | 268.146329876 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 466 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of moderate to severe vasomotor symptoms associated with the menopause, atrophic vaginitis, osteoporosis, hypoestrogenism due to hypogonadism, castration, primary ovarian failure, breast cancer (for palliation only), and Advanced androgen-dependent carcinoma of the prostate (for palliation only)
Equilin is a component of Premarin (conjugated estrogens), a mixture of the water soluble salts of sulfate esters from estrone, equilin, 17 alpha-dihydroequilin, and other related steroids, may be derived from pregnant equine urine or yam and soy plants. Estrogens are important in the development and maintenance of the female reproductive system and secondary sex characteristics. They promote growth and development of the vagina, uterus, and fallopian tubes, and enlargement of the breasts. Indirectly, they contribute to the shaping of the skeleton, maintenance of tone and elasticity of urogenital structures, changes in the epiphyses of the long bones that allow for the pubertal growth spurt and its termination, growth of axillary and pubic hair, and pigmentation of the nipples and genitals. Decline of estrogenic activity at the end of the menstrual cycle can bring on menstruation, although the cessation of progesterone secretion is the most important factor in the mature ovulatory cycle. However, in the preovulatory or nonovulatory cycle, estrogen is the primary determinant in the onset of menstruation. Estrogens also affect the release of pituitary gonadotropins. The pharmacologic effects of conjugated estrogens are similar to those of endogenous estrogens.
Absorption
Well absorbed.
Hepatic
Estrogens enter the cells of responsive tissues (e.g., female organs, breasts, hypothalamus, pituitary) where they interact with a protein receptor, subsequently increasing the rate of synthesis of DNA, RNA, and some proteins. Estrogens decrease the secretion of gonadotropin-releasing hormone by the hypothalamus, reducing the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary.
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
67
PharmaCompass offers a list of Equilin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Equilin manufacturer or Equilin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Equilin manufacturer or Equilin supplier.
PharmaCompass also assists you with knowing the Equilin API Price utilized in the formulation of products. Equilin API Price is not always fixed or binding as the Equilin Price is obtained through a variety of data sources. The Equilin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Equilin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Equilin, including repackagers and relabelers. The FDA regulates Equilin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Equilin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Equilin supplier is an individual or a company that provides Equilin active pharmaceutical ingredient (API) or Equilin finished formulations upon request. The Equilin suppliers may include Equilin API manufacturers, exporters, distributors and traders.
click here to find a list of Equilin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Equilin DMF (Drug Master File) is a document detailing the whole manufacturing process of Equilin active pharmaceutical ingredient (API) in detail. Different forms of Equilin DMFs exist exist since differing nations have different regulations, such as Equilin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Equilin DMF submitted to regulatory agencies in the US is known as a USDMF. Equilin USDMF includes data on Equilin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Equilin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Equilin suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Equilin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Equilin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Equilin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Equilin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Equilin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Equilin suppliers with NDC on PharmaCompass.
Equilin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Equilin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Equilin GMP manufacturer or Equilin GMP API supplier for your needs.
A Equilin CoA (Certificate of Analysis) is a formal document that attests to Equilin's compliance with Equilin specifications and serves as a tool for batch-level quality control.
Equilin CoA mostly includes findings from lab analyses of a specific batch. For each Equilin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Equilin may be tested according to a variety of international standards, such as European Pharmacopoeia (Equilin EP), Equilin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Equilin USP).
