
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
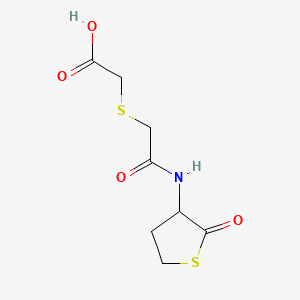

1. (+-)-((((tetrahydro-2-oxo-3-thienyl)carbamoyl)methyl)thio)acetic Acid
2. Dostein
3. Edirel
4. Esteclin
5. Rv 144
6. Rv-144
7. S-(2-(n-3-(2-oxo-tetrahydrothienyl)acetamido))thioglycolic Acid
8. Vectrine
1. 84611-23-4
2. Rv 144
3. Erdosteine [inn]
4. Mucotec
5. 105426-14-0
6. Rv-144
7. 2-[2-oxo-2-[(2-oxothiolan-3-yl)amino]ethyl]sulfanylacetic Acid
8. 2-((2-oxo-2-((2-oxotetrahydrothiophen-3-yl)amino)ethyl)thio)acetic Acid
9. 76j0853eka
10. Erdosteine (inn)
11. Kw-9144
12. Ncgc00185774-01
13. 2-({[(2-oxothiolan-3-yl)carbamoyl]methyl}sulfanyl)acetic Acid
14. Acetic Acid, [[2-oxo-2-[(tetrahydro-2-oxo-3-thienyl)amino]ethyl]thio]-
15. Dsstox_cid_28661
16. Dsstox_rid_82931
17. Dsstox_gsid_48735
18. Erdosteinum [latin]
19. Dithiosteine
20. Erdosteinum
21. Vectrine
22. Edirel
23. ({2-oxo-2-[(2-oxotetrahydro-3-thienyl)amino]ethyl}sulfanyl)acetic Acid
24. Acetic Acid, ((2-oxo-2-((tetrahydro-2-oxo-3-thienyl)amino)ethyl)thio)-
25. Cas-84611-23-4
26. Secresolv
27. Erdosteine [inn:ban]
28. Pv 144
29. Erdostiene
30. Erdotin
31. Unii-76j0853eka
32. 2-(2-oxo-2-(2-oxotetrahydrothiophen-3-ylamino)ethylthio)acetic Acid
33. Erdosteine,(s)
34. Mucotec (tn)
35. Erdosteine [mi]
36. Dl-s-(2-(n-3-(2-oxotetrahydrotheinyl)acetamido))thioglycolic Acid
37. ((2-oxo-2-((tetrahydro-2-oxo-3-thienyl)amino)ethyl)thio)acetic Acid
38. (+-)-((((tetrahydro-2-oxo-3-thienyl)carbamoyl)methyl)thio)acetic Acid
39. Acide ((2-oxo-3-tetrahydrothienylcarbamoyl)-methylthio)acetique [french]
40. Erdosteine [mart.]
41. Erdosteine [who-dd]
42. Schembl21721
43. Mls006010056
44. Chembl1697744
45. Dtxsid8048735
46. Erdosteine, >=98% (hplc)
47. Chebi:135014
48. Hms3264c11
49. Hms3655i14
50. Acide ((2-oxo-3-tetrahydrothienylcarbamoyl)-methylthio)acetique
51. Hy-b0289
52. Tox21_113176
53. Mfcd00867664
54. S1825
55. Stl452949
56. Akos015888361
57. Tox21_113176_1
58. Ac-5278
59. Ccg-213836
60. Db05057
61. Ks-1352
62. Ncgc00185774-02
63. Ncgc00185774-09
64. Smr002529560
65. E1026
66. Ft-0625684
67. Ft-0630929
68. Sw220302-1
69. D07383
70. T72990
71. Ab01274804-01
72. Ab01274804_02
73. Ab01274804_03
74. 611e234
75. A840878
76. Sr-01000944253
77. Q3731252
78. Sr-01000944253-1
79. N-(tetrahydro-2-oxo-3-thienyl)-3-thiapentanedioic Acid Monoamide
80. ({2-oxo-2-[(2-oxotetrahydrothiophen-3-yl)amino]ethyl}sulfanyl)acetic Acid
81. (+/-)-((((tetrahydro-2-oxo-3-thienyl)carbamoyl)methyl)thio)acetic Acid
82. 2-[2-oxo-2-[(2-oxotetrahydrothiophen-3-yl)amino]ethyl]sulfanylacetic Acid;erdosteine
83. Erdosteine; [[2-oxo-2-[(tetrahydro-2-oxo-3-thienyl)amino]ethyl]thio]acetic Acid
| Molecular Weight | 249.3 g/mol |
|---|---|
| Molecular Formula | C8H11NO4S2 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 249.01295018 g/mol |
| Monoisotopic Mass | 249.01295018 g/mol |
| Topological Polar Surface Area | 134 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 282 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Fro the treatment of chronic bronchitis in adults.
Expectorants
Agents that increase mucous excretion. Mucolytic agents, that is drugs that liquefy mucous secretions, are also included here. (See all compounds classified as Expectorants.)
R05CB15
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R05 - Cough and cold preparations
R05C - Expectorants, excl. combinations with cough suppressants
R05CB - Mucolytics
R05CB15 - Erdosteine
Erdosteine, is an orally administered mucolytic agent. It is classified as a thiol derivative and produced for the management of symptoms caused by chronic obstructive bronchitis. Erdosteine contains sulfhydryl groups which are released after hepatic first-pass metabolism in the liver. Its active metabolites (3 in number) exert both mucolytic activity and scavenging activity against free radicals. Erdosteine acts to regulate the production of mucus in the airway and regulates its viscosity while enhancing mucociliary transport. This leads to an increase in expectoration. Erdosteine shows inhibition against the effects of free radicals from cigarette smoke. Clinical studies in patients with chronic obstructive lung disease (COPD) have shown that this drug is generally safe and well tolerated. Erdosteine 300mg twice daily reduced cough (both frequency and severity) and sputum viscosity more quickly and more effectively than placebo and reduced the adhesivity of sputum more effectively than ambroxol 30mg twice daily. Co-administration of erdosteine and amoxicillin in patients with acute infective exacerbation of chronic bronchitis resulted in higher concentrations of the antibiotic in the sputum, leading to earlier and more pronounced amelioration of clinical symptoms compared with placebo. Erdosteine is associated with a low incidence of adverse events, most of which are gastrointestinal and generally mild.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

ABOUT THIS PAGE
35
PharmaCompass offers a list of Erdosteine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Erdosteine manufacturer or Erdosteine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Erdosteine manufacturer or Erdosteine supplier.
PharmaCompass also assists you with knowing the Erdosteine API Price utilized in the formulation of products. Erdosteine API Price is not always fixed or binding as the Erdosteine Price is obtained through a variety of data sources. The Erdosteine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Erdosteine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Erdosteine, including repackagers and relabelers. The FDA regulates Erdosteine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Erdosteine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Erdosteine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Erdosteine supplier is an individual or a company that provides Erdosteine active pharmaceutical ingredient (API) or Erdosteine finished formulations upon request. The Erdosteine suppliers may include Erdosteine API manufacturers, exporters, distributors and traders.
click here to find a list of Erdosteine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Erdosteine Drug Master File in Korea (Erdosteine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Erdosteine. The MFDS reviews the Erdosteine KDMF as part of the drug registration process and uses the information provided in the Erdosteine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Erdosteine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Erdosteine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Erdosteine suppliers with KDMF on PharmaCompass.
A Erdosteine written confirmation (Erdosteine WC) is an official document issued by a regulatory agency to a Erdosteine manufacturer, verifying that the manufacturing facility of a Erdosteine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Erdosteine APIs or Erdosteine finished pharmaceutical products to another nation, regulatory agencies frequently require a Erdosteine WC (written confirmation) as part of the regulatory process.
click here to find a list of Erdosteine suppliers with Written Confirmation (WC) on PharmaCompass.
Erdosteine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Erdosteine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Erdosteine GMP manufacturer or Erdosteine GMP API supplier for your needs.
A Erdosteine CoA (Certificate of Analysis) is a formal document that attests to Erdosteine's compliance with Erdosteine specifications and serves as a tool for batch-level quality control.
Erdosteine CoA mostly includes findings from lab analyses of a specific batch. For each Erdosteine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Erdosteine may be tested according to a variety of international standards, such as European Pharmacopoeia (Erdosteine EP), Erdosteine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Erdosteine USP).
