
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
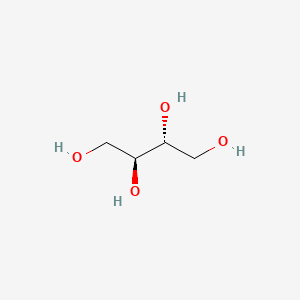

1. Meso-erythritol
2. 149-32-6
3. Phycitol
4. Erythrit
5. Mesoerythritol
6. Erythrite
7. Phycite
8. Erythrol
9. L-erythritol
10. (2r,3s)-butane-1,2,3,4-tetrol
11. Antierythrite
12. 1,2,3,4-butanetetrol, (2r,3s)-rel-
13. Erythro-tetritol
14. Butanetetrol
15. Erythroglucin
16. I-erythritol
17. Tetrahydroxybutane
18. Erythritol [nf]
19. 1,2,3,4-butanetetrol
20. Paycite
21. (2s,3r)-butane-1,2,3,4-tetrol
22. Chebi:17113
23. C*eridex
24. Nik 242
25. Meso-1,2,3,4-tetrahydroxybutane
26. Erythritol, Meso-
27. Erythritol,meso-erythritol
28. 10030-58-7
29. Nsc8099
30. Erythritol (nf)
31. Ra96b954x6
32. Erythrol (van)
33. Nsc-8099
34. (2r,3s)-butane-1,2,3,4-tetraol
35. Rel-(2r,3s)-butane-1,2,3,4-tetraol
36. 1,2,3,4-butanetetrol, (r*,s*)-
37. Lichen Sugar
38. (2r,3s)-rel-butane-1,2,3,4-tetraol
39. Nsc 8099
40. Mry
41. Smr000112220
42. Cargill Zerose 16957
43. Mfcd00004710
44. Meso-eythritol
45. Unii-ra96b954x6
46. Ccris 7901
47. Hsdb 7968
48. 1,2,3,4-butanetetrol, (theta,s)-
49. Einecs 205-737-3
50. L-(-)-threitol
51. D-erythritol
52. E968
53. Erythritol [mi]
54. Erythritol [fcc]
55. Erythritol [inci]
56. Wln: Q1yqyq1q
57. Erythritol [vandf]
58. 1,3,4-tetrahydroxybutane
59. Epitope Id:114707
60. F 8015
61. Meso-erythritol, >=99%
62. Erythritol [mart.]
63. Dsstox_cid_23919
64. Dsstox_rid_80090
65. Erythritol [usp-rs]
66. Dsstox_gsid_43919
67. Schembl17062
68. Mls001332365
69. Mls001332366
70. Zerose Tm 16957
71. Chembl349605
72. Ins No.968
73. Dtxsid6043919
74. Erythritol [ep Impurity]
75. Fema No. 4819
76. Erythritol [ep Monograph]
77. Ins-968
78. Hms2270m08
79. Pharmakon1600-01301025
80. Meso-erythritol, Analytical Standard
81. Tox21_200564
82. Nsc760400
83. S4224
84. Zinc17971067
85. 1,3,4-butanetetrol, (r*,s*)-
86. Akos006339851
87. Am83963
88. Ccg-266079
89. Db04481
90. Ds-5851
91. Nsc-760400
92. Ncgc00247033-01
93. Ncgc00258118-01
94. Cas-149-32-6
95. E-968
96. E0021
97. Sw219107-1
98. C00503
99. D08915
100. E70403
101. Wurcs=2.0/1,1,0/[h22h]/1/
102. Butane-1,2,3,4-tetrol, (2r,3s)-
103. 149e326
104. Butane 1,2,3,4-tetrol (meso-erythritol)
105. Q421873
106. F0001-2636
107. Bdf1567c-b08b-425a-b87f-15ff46328423
108. Erythritol, European Pharmacopoeia (ep) Reference Standard
109. Erythritol, United States Pharmacopeia (usp) Reference Standard
110. Erythritol, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 122.12 g/mol |
|---|---|
| Molecular Formula | C4H10O4 |
| XLogP3 | -2.3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 122.05790880 g/mol |
| Monoisotopic Mass | 122.05790880 g/mol |
| Topological Polar Surface Area | 80.9 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 48 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
In 12 male subjects who consumed 1 g/kg bw per day erythritol in a variety of foods during a five-day test period under controlled conditions, the mean urinary excretion was 61-88% of the nominal ingested dose, with an average of 78%.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
The peak serum concentration of erythritol in five non-insulin-dependent diabetic patients (sex not indicated) who consumed a single dose of 20 g erythritol in solution occurred 1 hr after administration and was 650 +/= 37 ug/mL. On average, 82, 88, and 88% of the administered erythritol was recovered in the urine 24, 48, and 72 hrs after dosing, respectively.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
After 12 male and 12 female volunteers received a dose of 0.4 or 0.8 g/kg bw erythritol in a chocolate snack, the plasma erythritol concentrations increased rapidly, reaching peaks of 3 and 5 mmol/L 1 and 2 hrs after dosing with 0.4 and 0.8 g/kg bw, respectively. Starting 2 hrs after treatment, the plasma erythritol concentrations were significantly (p < 0.05) higher in the group given 0.8 g/kg bw than in that given 0.4 g/kg bw. At both doses, erythritol appeared in the urine within 2 hrs of dosing, the largest quantities being collected between 2 and 4 hrs after administration. Erythritol was still present in urine 22 hrs after treatment. The concentration of erythritol in the urine of individuals given 0.8 g/kg bw was about twice and significantly (p < 0.05) greater than that in the urine of people given 0.4 g/kg bw. On average, 61 and 62% of the administered erythritol was recovered in the urine after the 0.4 and 0.8 g/kg bw doses, respectively, within 22 hrs.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
The kinetics of erythritol in plasma and urine were investigated in three men and three women after an overnight fast. Each subject ingested a single oral dose of 1 g/kg bw dissolved in 250 mL of water, and blood samples were taken 5, 10, 15, 30, 45, 60, 90, 120, 180, and 240 min after dosing for determination of plasma concentrations of erythritol. Plasma creatinine concentrations were determined in a blood sample taken before treatment. Urine was collected over 0-30 min, 30-60 min, 1-2 hr, 2-3 hr, and 3-24 hr after treatment for determination of the volume and of the erythritol and creatinine concentrations. Erythritol was detected in the plasma 10 min after dosing, and the mean plasma concentrations increased steadily from 15 min after treatment to a peak of 2.2 mg/mL after 90 min. The urine volume and erythritol concentration reached a maximum during 1-2 hr after ingestion, at about the same time that the plasma concentration of erythritol peaked. Urinary recovery of erythritol over the 24 hr collection period accounted for 78% of the administered dose, with 30% collected after 3 hr. During 1 and 2 hr after administration, the clearance of erythritol was about half that of creatinine, indicating tubular reabsorption of erythritol by the kidney.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for Erythritol (20 total), please visit the HSDB record page.
In studies designed to investigate the metabolism of erythritol in vivo in healthy volunteers and to compare the fermentation of erythritol by human fecal flora in vitro with that of glucose and lactitol, four male and two female volunteers aged 21-25 undertook an overnight fast and were then chosen at random to receive a single dose of 25 g (13)C-erythritol, (13)C-glucose, and (13)C-lactitol in 250 mL of water with at least three days between each treatment. Breath samples were taken for analysis of (13)C-carbon dioxide and hydrogen gas before treatment and at 30 min intervals up to 6 hr after treatment. The ratio of (13)C:(12)C-carbon dioxide was measured by isotope-ratio mass spectrometry. ... In order to maintain a constant metabolic rate, the subjects remained at rest during the study. For the assay of fermentation in vitro, fecal samples were collected from six healthy volunteers (sex and age not specified) who ate a normal western diet. None of the subjects complained of gastrointestinal symptomsand none had used antibiotics in the past six months. The samples were incubated under anaerobic conditions for 6 hr, and then the hydrogen gas concentration was measured in the head-space of the incubation vials. ... After a 6 hr incubation with erythritol, the amount of hydrogen gas formed by the fecal flora was comparable to that in control vials, but significantly (p < 0.001) more hydrogen gas was produced in the glucose and lactitol vials than in either control or erythritol.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
Groups of three Wistar rats of each sex were given a single dose of 0.1 g/kg bw (14)C-erythritol by gavage, as follows: germ-free rats were kept under sterile conditions until administration of commercial (14)C-erythritol; adapted conventional rats received diets containing weekly increases of 5, 10, and 20% erythritol for three weeks before administration of commercial (14)C-erythritol; unadapted conventional rats were kept on CIVO stock diet before administration of commercial (14)C-erythritol; or germ-free rats were kept under sterile conditions until dosing with purified (14)C-erythritol. Rats were not fasted before dosing. Immediately after treatment, the rats were placed in individual metabolism cages to allow collection of expired carbon dioxide, urine, and feces over 24 hrs. ... In rats that received commercial (14)C-erythritol, 2.5-2.9% of the radiolabel was erythrose and 0.2-0.35% was glucose. In germ-free rats that received purified (14)C-erythritol, less radiolabel was associated with erythrose. No volatile radioactive components were identified by lyophilization of the urine samples.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
Groups of 11 male Wistar rats were fed control diet or control diet containing 10% erythritol (added at the expense of corn starch) for two weeks. They were then sacrificed, the caecal contents were collected and pooled by group, and the contents were suspended. Samples of each suspension were incubated with 12 mg (14)C-erythritol (10 uCi) for 6 hr under anaerobic conditions, and the incubation mixture was analyzed for erythritol, short-chain fatty acids, and carbon dioxide 1, 2, 4, and 6 hr after the beginning of incubation. The total recoveries of radiolabel were comparable for control and treated groups at the end of incubation, but the proportions of all (14)C-labelled products of fermentation differed significantly ( p < 0.01) between the two groups: in the controls, 84% of the radiolabel was present as unchanged erythritol, and carbon dioxide, acetic acid, propionic acid, and butyric acid each accounted for < 2% of the radiolabel; in contrast, < 1% of the radiolabel in the caecal contents of treated rats was present as erythritol at the end of incubation, 17% of the administered dose was released as (14)C-carbon dioxide within 2 hr of incubation, and 24% of the radiolabel was recovered as (14)C-carbon dioxide by the end of the incubation period. Succinic, acetic, propionic, and butyric acids were identified as fermentation products and accounted for about 60% of the radiolabel at the end of the incubation period. Succinic acid was detectable after 1 hr but not subsequently, suggesting that it was fermented to other products.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
After a 12 hr fast, five men received a single oral dose of 0.3 g/kg bw erythritol as a 20% aqueous solution. Urine samples were collected over 48 hr and blood samples over 24 hr to allow determination of erythritol. The compound appeared to be readily absorbed, the plasma concentration peaking at 430 g/mL 30 min after treatment, with a half-time of 3.4 hr. ...
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
Three beagle dogs were given a single oral dose of 1 g/kg bw (14)C-erythritol after an 18 hr fast and were then housed individually in metabolism cages and fasted for an additional 8 hr. Blood samples were collected from each dog 1, 5, 15, and 30 min and 1, 2, 3, 4, 5, 6, 8, 24, 48, 72, 96, and 120 hr after dosing for determination of radiolabel in blood and plasma and for calculation of the ratio of distribution to erythrocytes. ... The concentration of erythritol in blood and plasma reached a peak 30 min after dosing and decreased in a biphasic manner with half-times of 0.07 and 1.7 hr in blood and 0.5 and 5.4 hr in plasma. ...
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
Groups of three male Wistar rats were given a single oral dose of 0.125, 0.25, 0.5, 1, or 2 g (14)C-erythritol/kg bw after an 18 hr fast, and they were fasted for an additional 8 hr after dosing. Blood samples were collected from the tail vein 15 and 30 min and 1, 2, 4, 6, 8, 12, 24, 48, and 72 hr after dosing in order to measure radioactivity. ... After the initial absorption, a biphasic decrease in the blood concentration of radiolabel was seen, with half-time values for the biphasic decrease of about 2 and 20 hr, respectively. The biphasic decrease was also observed at 2 g/kg bw, but the half-time values were longer (3 and 28 hr), indicating a slower rate of elimination. ...
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 44: Erythritol (149-32-6) (2000). Available from, as of July 12, 2011: https://www.inchem.org/pages/jecfa.html
... Reports from authoritative bodies and reviews indicates that the decrease in pH in plaque as a consequence of metabolic acid production by saccharolytic bacteria when exposed to fermentable carbohydrates (i.e. sugars and starches) may promote demineralization and prevent remineralization of the hydroxyapatite crystals. Tooth hydroxyapatite crystals are very resistant to dissolution at neutral pH, but their solubility drastically increases as pH drops. Typically, the critical pH for dental enamel is around 5.5. ... Demineralization of tooth tissues can also occur as a result of consumption of dietary acids in foods or beverages, and that frequent consumption can lead to dental erosion. Xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose are slowly metabolized by bacteria in the mouth. The rate and amount of acid production from these food constituents is significantly less than that from sucrose. ... Xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose do not promote dental caries because they do not lower plaque pH to the level associated with enamel demineralization. ... A cause and effect relationship has been established between the consumption of sugar-containing foods/drinks at an exposure frequency of four times daily or more and an increased tooth demineralization, and that the consumption of foods/drinks containing xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose, instead of sugar in sugar-containing foods/drinks, may maintain tooth mineralization by decreasing tooth demineralization compared with sugar-containing foods, provided that such foods/drinks do not lead to dental erosion.
European Food Safety Authority (EFSA); EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation and reduction of post-prandial glycaemic responses (April 2011). Available from, as of July 28, 2011: https://www.efsa.europa.eu/en/publications.htm
The food constituents xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose resulted in reduced post-prandial blood glucose (or insulinemic) responses compared with sugars on a weight by weight basis owing to their reduced/delayed digestion/absorption and/or to a decrease in the amount of available carbohydrates, and that the consumption of foods/drinks in which xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose replaced sugars induced lower post-prandial glycaemic and insulinaemic responses than sugar-containing foods/drinks. ... A cause and effect relationship has been established between the consumption of foods/drinks containing xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose instead of sugar and reduction in post-prandial blood glucose responses (without disproportionally increasing post-prandial insulinemic responses) as compared to sugar-containing foods/drinks.
European Food Safety Authority (EFSA); EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation and reduction of post-prandial glycaemic responses (April 2011). Available from, as of July 28, 2011: https://www.efsa.europa.eu/en/publications.htm

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
16
PharmaCompass offers a list of Meso-Erythritol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Meso-Erythritol manufacturer or Meso-Erythritol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Meso-Erythritol manufacturer or Meso-Erythritol supplier.
PharmaCompass also assists you with knowing the Meso-Erythritol API Price utilized in the formulation of products. Meso-Erythritol API Price is not always fixed or binding as the Meso-Erythritol Price is obtained through a variety of data sources. The Meso-Erythritol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Erythritol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Erythritol, including repackagers and relabelers. The FDA regulates Erythritol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Erythritol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Erythritol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Erythritol supplier is an individual or a company that provides Erythritol active pharmaceutical ingredient (API) or Erythritol finished formulations upon request. The Erythritol suppliers may include Erythritol API manufacturers, exporters, distributors and traders.
click here to find a list of Erythritol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Erythritol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Erythritol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Erythritol GMP manufacturer or Erythritol GMP API supplier for your needs.
A Erythritol CoA (Certificate of Analysis) is a formal document that attests to Erythritol's compliance with Erythritol specifications and serves as a tool for batch-level quality control.
Erythritol CoA mostly includes findings from lab analyses of a specific batch. For each Erythritol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Erythritol may be tested according to a variety of international standards, such as European Pharmacopoeia (Erythritol EP), Erythritol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Erythritol USP).
