
Synopsis
0
Canada

0
Australia

0
South Africa

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
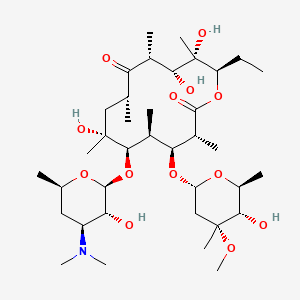

1. Erycette
2. Erymax
3. Erythromycin A
4. Erythromycin C
5. Erythromycin Lactate
6. Erythromycin Phosphate
7. Ilotycin
8. Lactate, Erythromycin
9. Phosphate, Erythromycin
10. T Stat
11. T-stat
12. Tstat
1. 114-07-8
2. Erythromycin A
3. E-mycin
4. Ilotycin
5. Abomacetin
6. Erymax
7. Erythromycinum
8. Emgel
9. Erycette
10. Robimycin
11. Erythro-statin
12. E-glades
13. Erythrocin
14. Ery-tab
15. E-base
16. Eritromicina
17. Erythromycine
18. Erygel
19. Sansac
20. Erythra-derm
21. Akne-mycin
22. Eryc
23. Erythromycin Base
24. T-stat
25. Eryacne
26. R-p Mycin
27. E-solve 2
28. Torlamicina
29. Eryderm
30. C-solve-2
31. Pantomicina
32. A/t/s
33. Erythro
34. Erythroguent
35. Eritrocina
36. Propiocine
37. Stiemycin
38. Ermycin
39. Erycen
40. Aknin
41. Theramycin Z
42. Erythromycin-a
43. Ak-mycin
44. Erythromast 36
45. (-)-erythromycin
46. Benzamycin
47. Emycin
48. Erytab
49. Inderm
50. Retcin
51. Chembl532
52. Dumotrycin
53. Mephamycin
54. Chebi:42355
55. Wemid
56. J01fa01
57. Eryc Sprinkles
58. Mfcd00084654
59. Nsc-55929
60. 63937kv33d
61. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-(((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-14-ethyl-7,12,13-trihydroxy-4-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione
62. Erythromycin C-13
63. Endoeritrin
64. Eritomicina
65. Erythroderm
66. Erytrociclin
67. Pharyngocin
68. Proterytrin
69. Acneryne
70. Acnesol
71. Aknemycin
72. Derimer
73. Deripil
74. Erisone
75. Eryacnen
76. Erydermer
77. Eryhexal
78. Erysafe
79. Iloticina
80. Latotryd
81. Lederpax
82. Mercina
83. Oftamolets
84. Pantoderm
85. Pantodrin
86. Primacine
87. Romycin
88. Stiemicyn
89. Tiprocin
90. Emuvin
91. Erecin
92. Erymed
93. Erytop
94. Nci-c55674
95. Austrias
96. Eros
97. Ery-maxin
98. Erythro-teva
99. Sans-acne
100. Dsstox_cid_2991
101. Erimycin-t
102. Ery-diolan
103. Inderm Gel
104. Del-mycin
105. Aknederm Ery Gel
106. Udima Ery Gel
107. Eryc 125
108. Dsstox_rid_76820
109. Dsstox_gsid_22991
110. Emu-ve
111. 8hph7nd0ln
112. Skid Gel E
113. (3r*,4s*,5s*,6r*,7r*,9r*,11r*,12r*,13s*,14r*)-4-((2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy)-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-((3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl)oxy)oxacyclotetradecane-2,10-dione
114. Akne Cordes Losung
115. Ery
116. Ilosone (estolate)
117. Ilotycin T.s.
118. E-mycin (base)
119. Ery-tab (base)
120. Emu-v
121. Ery-b
122. E-base (base)
123. Eryc (base)
124. N-methylerythromycin A
125. Pce Dispertab (base)
126. Eryc-125
127. Eryc-250
128. Eritromicina [inn-spanish]
129. Erythromycine [inn-french]
130. Erythromycinum [inn-latin]
131. Erythromycin, Labeled With Carbon-13
132. Oftalmolosa Cusi Eritromicina
133. Staticin (tn)
134. Akne-mycin (tn)
135. Erygel (tn)
136. Eryc (tn)
137. T-stat (tn)
138. Pce (tn)
139. Sr-05000001618
140. Sentry Aq Mardel Maracyn
141. 9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione
142. Erthromycin
143. Erythromycines
144. Nsc55929
145. Ccris 9078
146. Unii-63937kv33d
147. Hsdb 3074
148. Ncgc00094670-01
149. Erythromycin [usp:inn:ban:jan]
150. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,7,
151. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-{[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione
152. Cas-114-07-8
153. Prestwick_205
154. Einecs 204-040-1
155. Nsc 55929
156. 215031-94-0
157. Spectrum_000115
158. Spectrum_000659
159. Ai3-50138
160. Em-a
161. Prestwick3_000151
162. Spectrum2_000759
163. Spectrum2_001263
164. Spectrum4_000538
165. Spectrum5_001596
166. Unii-8hph7nd0ln
167. Erythromycin [mi]
168. E0751
169. Erythromycin [inn]
170. Erythromycin [jan]
171. Pce (erythromycin)
172. Ec 204-040-1
173. Erythromycin [hsdb]
174. Schembl2601
175. Erythromycin [vandf]
176. Bspbio_000282
177. Bspbio_002480
178. Erythromycin [mart.]
179. Erythromycin Standard Solution
180. Kbiogr_001175
181. Kbioss_000555
182. Kbioss_001139
183. 82343-12-2
184. Mls001066618
185. Bidd:gt0017
186. Divk1c_000294
187. Divk1c_000397
188. Divk1c_000702
189. Erythromycin [usp-rs]
190. Erythromycin [who-dd]
191. Erythromycin [who-ip]
192. Spectrum1500280
193. Spbio_000778
194. Spbio_001226
195. Bpbio1_000312
196. Gtpl1456
197. Dtxsid4022991
198. Erythromycin (jp17/usp/inn)
199. Hms500o16
200. Kbio1_000294
201. Kbio1_000397
202. Kbio1_000702
203. Kbio2_000555
204. Kbio2_001139
205. Kbio2_003123
206. Kbio2_003707
207. Kbio2_005691
208. Kbio2_006275
209. Erythromycin [green Book]
210. Erythromycin (mixture Of A,b,c)
211. Ninds_000294
212. Ninds_000397
213. Ninds_000702
214. Erythromycin [orange Book]
215. Hms1920m04
216. Hms2091d05
217. Hms2095o04
218. Hms3712o04
219. Pharmakon1600-01500280
220. Erythromycin [ep Monograph]
221. Act03320
222. Hy-b0220
223. Rkl10096
224. Erythromycin [usp Monograph]
225. Tox21_111311
226. Tox21_111869
227. Tox21_300515
228. Bdbm50344942
229. Ccg-38992
230. Lmpk04000006
231. Nsc756759
232. Zinc85534336
233. Erythromycinum [who-ip Latin]
234. Akos015895249
235. Db00199
236. Nsc-756759
237. Benzamycin Component Erythromycin
238. Idi1_000294
239. Idi1_000397
240. Idi1_000702
241. Smp1_000119
242. Ncgc00179619-01
243. Ncgc00179619-02
244. Ncgc00179619-03
245. Ncgc00254234-01
246. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione
247. Ac-12744
248. Ac-12901
249. Smr000544946
250. Erythromycin Component Of Benzamycin
251. Erythromycin, Potency: >=850 Mug Per Mg
252. Erythromycin, Tested According To Ph.eur.
253. Sbi-0051368.p003
254. Erythromycin, N-demethyl-n-(methyl-11c)-
255. Erythromycin 1000 Microg/ml In Acetonitrile
256. Erythromycin, Meets Usp Testing Specifications
257. C01912
258. D00140
259. E-3250
260. Erythromycin, Plant Cell Culture Tested, ~98%
261. Ab00051981_09
262. Ab00051981_10
263. Erythromycin Standard Solution, 1 Mg/ml In H2o
264. Erythromycin, Biotechnology Performance Certified
265. 114e078
266. Erythromycin Estolate Impurity, Free Erythromycin-
267. Q213511
268. Sr-01000799155
269. Erythromycin, Antibiotic For Culture Media Use Only
270. Erythromycin, Bioreagent, Suitable For Cell Culture
271. Sr-01000799155-2
272. Sr-05000001618-1
273. Sr-05000001618-2
274. Brd-k63550407-001-13-5
275. Brd-k63550407-028-03-9
276. Erythromycin (mixture Of A,b,c) 100 Microg/ml In Acetonitrile
277. Erythromycin A, British Pharmacopoeia (bp) Reference Standard
278. Erythromycin A, European Pharmacopoeia (ep) Reference Standard
279. Erythromycin, United States Pharmacopeia (usp) Reference Standard
280. Erythromycin Estolate Impurity, Free Erythromycin [ep Impurity]
281. Erythromycin Estolate Impurity, Free Erythromycin- [usp Impurity]
282. Erythromycin, For Microbiological Assay, European Pharmacopoeia (ep) Reference Standard
283. Erythromycin, Pharmaceutical Secondary Standard; Certified Reference Material
284. (3r*,4s*,5s*,6r*,7r*,9r*,11r*,12r*,13s*,14r*)-4-[(2,6-dideoxy-3-c-methyl-3-o-methyl-.alpha.-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-
285. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-4-(2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyloxy)-14-ethyl-7,12,13-trihydroxy-6-[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyloxy]-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione
286. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyl-tetrahydropyran-2-yl]oxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione
287. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-dimethylamino-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione
288. (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-{[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione (non-preferred Name)
289. [3r-(3r*,4s*,5s*,6r*,7r*,9r*,11r*,12r*,13s*,14r*)]-4-[(2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione
| Molecular Weight | 733.9 g/mol |
|---|---|
| Molecular Formula | C37H67NO13 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 7 |
| Exact Mass | 733.46124119 g/mol |
| Monoisotopic Mass | 733.46124119 g/mol |
| Topological Polar Surface Area | 194 Ų |
| Heavy Atom Count | 51 |
| Formal Charge | 0 |
| Complexity | 1180 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 24 | |
|---|---|
| Drug Name | Akne-mycin |
| Active Ingredient | Erythromycin |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Dow Pharm |
| 2 of 24 | |
|---|---|
| Drug Name | Benzamycin |
| Active Ingredient | Benzoyl peroxide; erythromycin |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 5%; 3% |
| Market Status | Prescription |
| Company | Valeant Intl |
| 3 of 24 | |
|---|---|
| Drug Name | Benzamycin pak |
| Drug Label | ERYC capsules contain enteric-coated pellets of erythromycin base for oral administration. Each ERYC capsule contains 250 mg of erythromycin base. Also contains: lactose NF, povidone USP, FD&C Yellow #6 and other ingredients. The capsule shell contai. |
| Active Ingredient | Benzoyl peroxide; erythromycin |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 5%; 3% |
| Market Status | Prescription |
| Company | Valeant Luxembourg |
| 4 of 24 | |
|---|---|
| Drug Name | C-solve-2 |
| Active Ingredient | Erythromycin |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 5 of 24 | |
|---|---|
| Drug Name | Eryc |
| Active Ingredient | Erythromycin |
| Dosage Form | Capsule, delayed rel pellets |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Mayne Pharma |
| 6 of 24 | |
|---|---|
| Drug Name | Erygel |
| PubMed Health | Erythromycin (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | ERYGEL Topical Gel USP, 2% contains erythromycin (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl--L-ribo-hexopyranosyl)oxy]-14-ethyl-7, 12, 13-trihydroxy-3, 5, 7, 9, 11, 13-hexamethyl-6-[[3, 4, 6,-trid... |
| Active Ingredient | Erythromycin |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Delcor Asset |
| 7 of 24 | |
|---|---|
| Drug Name | Ery-tab |
| PubMed Health | Erythromycin |
| Drug Classes | Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic |
| Drug Label | ERY-TAB (erythromycin delayed-release tablets) is an antibacterial product containing erythromycin base in a specially enteric-coated tablet to protect it from the inactivating effects of gastric acidity and to permit efficient absorption of the anti... |
| Active Ingredient | Erythromycin |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 250mg; 333mg; 500mg |
| Market Status | Prescription |
| Company | Arbor Pharms |
| 8 of 24 | |
|---|---|
| Drug Name | Erythra-derm |
| Drug Label | Erythromycin is an antibiotic produced from a strain of Streptomyces erythraeus. It is basic and readily forms salts with acids.Chemically, erythromycin is (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6-Dideoxy-3-C-methyl-3-0-methyl-... |
| Active Ingredient | Erythromycin |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Paddock |
| 9 of 24 | |
|---|---|
| Drug Name | Erythrocin |
| PubMed Health | Erythromycin/Benzoyl Peroxide (On the skin) |
| Drug Classes | Antiacne |
| Active Ingredient | Erythromycin lactobionate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Hospira |
| 10 of 24 | |
|---|---|
| Drug Name | Erythrocin stearate |
| Active Ingredient | Erythromycin stearate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 250mg base |
| Market Status | Prescription |
| Company | Arbor Pharms |
| 11 of 24 | |
|---|---|
| Drug Name | Erythromycin |
| Drug Label | ERY-TAB (erythromycin delayed-release tablets) is an antibacterial product containing erythromycin base in a specially enteric-coated tablet to protect it from the inactivating effects of gastric acidity and to permit efficient absorption of the anti... |
| Active Ingredient | Erythromycin |
| Dosage Form | Gel; Swab; Capsule, delayed rel pellets; Ointment; Tablet; Solution |
| Route | Topical; Ophthalmic; Oral |
| Strength | 0.5%; 250mg; 2%; 500mg |
| Market Status | Prescription |
| Company | Arbor Pharms; Wockhardt; Fougera Pharms; Perrigo Co Tennessee; Versapharm; Bausch And Lomb; Perrigo; Perrigo New York; Akorn |
| 12 of 24 | |
|---|---|
| Drug Name | Erythro-statin |
| Active Ingredient | Erythromycin |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Hi Tech Pharma |
| 13 of 24 | |
|---|---|
| Drug Name | Akne-mycin |
| Active Ingredient | Erythromycin |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Dow Pharm |
| 14 of 24 | |
|---|---|
| Drug Name | Benzamycin |
| Active Ingredient | Benzoyl peroxide; erythromycin |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 5%; 3% |
| Market Status | Prescription |
| Company | Valeant Intl |
| 15 of 24 | |
|---|---|
| Drug Name | Benzamycin pak |
| Drug Label | ERYC capsules contain enteric-coated pellets of erythromycin base for oral administration. Each ERYC capsule contains 250 mg of erythromycin base. Also contains: lactose NF, povidone USP, FD&C Yellow #6 and other ingredients. The capsule shell contai. |
| Active Ingredient | Benzoyl peroxide; erythromycin |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 5%; 3% |
| Market Status | Prescription |
| Company | Valeant Luxembourg |
| 16 of 24 | |
|---|---|
| Drug Name | C-solve-2 |
| Active Ingredient | Erythromycin |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 17 of 24 | |
|---|---|
| Drug Name | Eryc |
| Active Ingredient | Erythromycin |
| Dosage Form | Capsule, delayed rel pellets |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Mayne Pharma |
| 18 of 24 | |
|---|---|
| Drug Name | Erygel |
| PubMed Health | Erythromycin (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | ERYGEL Topical Gel USP, 2% contains erythromycin (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl--L-ribo-hexopyranosyl)oxy]-14-ethyl-7, 12, 13-trihydroxy-3, 5, 7, 9, 11, 13-hexamethyl-6-[[3, 4, 6,-trid... |
| Active Ingredient | Erythromycin |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Delcor Asset |
| 19 of 24 | |
|---|---|
| Drug Name | Ery-tab |
| PubMed Health | Erythromycin |
| Drug Classes | Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic |
| Drug Label | ERY-TAB (erythromycin delayed-release tablets) is an antibacterial product containing erythromycin base in a specially enteric-coated tablet to protect it from the inactivating effects of gastric acidity and to permit efficient absorption of the anti... |
| Active Ingredient | Erythromycin |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 250mg; 333mg; 500mg |
| Market Status | Prescription |
| Company | Arbor Pharms |
| 20 of 24 | |
|---|---|
| Drug Name | Erythra-derm |
| Drug Label | Erythromycin is an antibiotic produced from a strain of Streptomyces erythraeus. It is basic and readily forms salts with acids.Chemically, erythromycin is (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6-Dideoxy-3-C-methyl-3-0-methyl-... |
| Active Ingredient | Erythromycin |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Paddock |
| 21 of 24 | |
|---|---|
| Drug Name | Erythrocin |
| PubMed Health | Erythromycin/Benzoyl Peroxide (On the skin) |
| Drug Classes | Antiacne |
| Active Ingredient | Erythromycin lactobionate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Hospira |
| 22 of 24 | |
|---|---|
| Drug Name | Erythrocin stearate |
| Active Ingredient | Erythromycin stearate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 250mg base |
| Market Status | Prescription |
| Company | Arbor Pharms |
| 23 of 24 | |
|---|---|
| Drug Name | Erythromycin |
| Drug Label | ERY-TAB (erythromycin delayed-release tablets) is an antibacterial product containing erythromycin base in a specially enteric-coated tablet to protect it from the inactivating effects of gastric acidity and to permit efficient absorption of the anti... |
| Active Ingredient | Erythromycin |
| Dosage Form | Gel; Swab; Capsule, delayed rel pellets; Ointment; Tablet; Solution |
| Route | Topical; Ophthalmic; Oral |
| Strength | 0.5%; 250mg; 2%; 500mg |
| Market Status | Prescription |
| Company | Arbor Pharms; Wockhardt; Fougera Pharms; Perrigo Co Tennessee; Versapharm; Bausch And Lomb; Perrigo; Perrigo New York; Akorn |
| 24 of 24 | |
|---|---|
| Drug Name | Erythro-statin |
| Active Ingredient | Erythromycin |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Hi Tech Pharma |
Antibiotics, Macrolide; Gastrointestinal Agents; Protein Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): In veterinary medicine, /erythromycin/ is used the treatment of clinical and subclinical mastitis in lactating cows, for the treatment of infectious diseases due to erythromycin-sensitive bacteria (cattle, sheep, swine, poultry) and for the treatment of chronic respiratory diseases due to mycoplasma in poultry.
Erythromycin is used as an alternative agent in the treatment of anthrax. Parenteral penicillins generally have been considered the drugs of choice for the treatment of naturally occurring or endemic anthrax caused by susceptible strains of Bacillus anthracis, including clinically apparent GI, inhalational, or meningeal anthrax and anthrax septicemia, although IV ciprofloxacin or IV doxycycline also are recommended. Erythromycin is suggested as an alternative to penicillin G for the treatment of naturally occurring or endemic anthrax in patients hypersensitive to penicillins. ./NOT included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 228
Erythromycin is used topically in the treatment of acne vulgaris. Therapy of acne vulgaris must be individualized and frequently modified depending on the types of acne lesions which predominate and the response to therapy. Topical anti-infectives, including erythromycin, are generally effective in the treatment of mild to moderate inflammatory acne. However, use of topical anti-infectives as monotherapy may lead to bacterial resistance; this resistance is associated with decreased clinical efficacy. Topical erythromycin is particularly useful when used with benzoyl peroxide or topical retinoids. Results of clinical studies indicate that combination therapy results in a reduction in total lesion counts of 50 to 70%. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3511
For more Therapeutic Uses (Complete) data for Erythromycin (23 total), please visit the HSDB record page.
Some commercially available formulations of erythromycin lactobionate powder for injection contain benzyl alcohol as a preservative. Although a causal relationship has not been established, administration of injections preserved with benzyl alcohol has been associated with toxicity in neonates. Toxicity appears to have resulted from administration of large amounts (i.e., about 100-400 mg/kg daily) of benzyl alcohol in these neonates. Although use of drugs preserved with benzyl alcohol should be avoided in neonates whenever possible, the American Academy of Pediatrics states that the presence of small amounts of the preservative in a commercially available injection should not proscribe its use when indicated in neonates. /Erythromycin lactobionate/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 240
In several neonates with infections caused by Ureaplasma urealyticum who received IV administration of erythromycin lactobionate, adverse cardiac effects (e.g., bradycardia, hypotension, cardiac arrest, arrhythmias) requiring cardiopulmonary resuscitation have been reported. Some clinicians state that these adverse effects may depend on serum concentration and/or infusion rate of the drug. It has been suggested that prolonged IV infusion of erythromycin lactobionate (e.g., over 60 minutes) may reduce such adverse cardiac effects. However, it has been suggested that certain individuals may be at increased risk of developing erythromycin-induced adverse cardiac effects and that decreasing the rate of IV infusion may decrease but not eliminate the risk of such effects. Further study is needed to determine the pharmacokinetics and safety of erythromycin lactobionate in neonates. /Erythromycin lactobionate/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 240
Maternal Medication usually Compatible with Breast-Feeding: Erythromycin: Reported Sign or Symptom in Infant or Effect on Lactation: None. /From Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 140 (1994)
POTENTIAL ADVERSE EFFECTS ON FETUS: None known. POTENTIAL SIDE EFFECTS ON BREAST-FED INFANT: None known, although theoretically could cause diarrhea in infant. COMMENTS: Crosses placenta in high doses to fetal level 24% of maternal; breast milk may exceed maternal serum concentration. FDA Category: B (B = Studies in laboratory animals have not demonstrated a fetal risk, but there are no controlled studies in pregnant women; or animal studies have shown an adverse effect (other than a decrease in fertility), but controlled studies in pregnant women have not demonstrated a risk to the fetus in the first trimester and there is no evidence of a risk in later trimesters.) /From Table II/
PMID:2195076 Stockton DL and AS Paller; J Am Acad Dermatol 23 (1): 87-103 (1990)
For more Drug Warnings (Complete) data for Erythromycin (17 total), please visit the HSDB record page.
Erythromycin is indicated in the treatment of infections caused by susceptible strains of various bacteria. The indications for erythromycin have been summarized by body system below: **Respiratory infections** Mild to moderate upper respiratory tract infections caused by Streptococcus pyogenes, Streptococcus pneumoniae, or Haemophilus influenzae (when used concomitantly with appropriate doses of sulfonamides) can be treated with erythromycin. Mild to moderate lower-respiratory tract infections due to susceptible strains of Streptococcus pneumoniae or Streptococcus pyogenes may also be treated. Erythromycin treats listeriosis caused by Listeria monocytogenes may also be treated with erythromycin. Erythromycin is indicated to treat pertussis (whooping cough) caused by Bordetella pertussis. It is effective in eliminating the causative organism from the nasopharynx of infected individuals, rendering them noninfectious. Clinical studies suggest that erythromycin may aid in the prevention of pertussis infection for individuals who have been exposed to the bacteria. Respiratory tract infections due to Mycoplasma pneumoniae may also be treated with erythromycin. Despite the fact that no controlled clinical efficacy studies have been conducted to this date, in vitro and certain preliminary clinical study results indicate that erythromycin may be an effective treatment in Legionnaires Disease. Finally, erythromycin is indicated to treat diphtheria and other infections due to Corynebacterium diphtheriae, as an adjunct to antitoxin, to prevent carrier status and to eradicate the organism in existing carriers. In addition to the prevention of diphtheria, erythromycin can be used to prevent rheumatic fever in penicillin intolerant patients. **Skin infections** Mild to moderate skin or skin structure infections caused by Streptococcus pyogenes or Staphylococcus aureus may be treated with erythromycin, however, resistant staphylococcal organisms may emerge. Erythromycin can also be used to treat erythrasma, an infectious condition caused by Corynebacterium minutissimum. **Gastrointestinal infections** Intestinal amebiasis caused by Entamoeba histolytica can be treated with oral erythromycin. Extraenteric amebiasis warrants treatment with other antimicrobial drugs. **Genital infections/STIs** Erythromycin can be used as an alternative drug in treating acute pelvic inflammatory disease caused by N. gonorrheae in female patients who have demonstrated hypersensitivity or intolerance to penicillin. Syphilis, caused by Treponema pallidum, can be treated with erythromycin. It serves as an alternative treatment for primary syphilis in patients who have demonstrated penicillin hypersensitivity. Erythromycin can also be used in the primary stage of primary syphilis. Another approved indication of erythromycin is to treat chlamydial infections that cause conjunctivitis of the newborn, pneumonia of infancy, and urogenital infections occurring in pregnancy. It is indicated as an alternative option to tetracyclines for the treatment of uncomplicated rectal, urethral and endocervical infections in adults caused by Chlamydia trachomatis. Erythromycin can be used in nongonococcal urethritis can be used when tetracyclines cannot be administered. Finally, erythromycin is indicated to treat nongonococcal urethritis due to Ureaplasma urealyticum.
Macrolides, such as erythromycin, stop bacterial growth by inhibiting protein synthesis and translation, treating bacterial infections. Erythromycin does not exert effects on nucleic acid synthesis. This drug has been shown to be active against most strains of the following microorganisms, effectively treating both in vitro and clinical infections. Despite this, it is important to perform bacterial susceptibility testing before administering this antibiotic, as resistance is a common issue that may affect treatment. **A note on antimicrobial resistance, pseudomembranous colitis, and hepatotoxicity** Many strains of Haemophilus influenzae are resistant to erythromycin alone but are found to be susceptible to erythromycin and sulfonamides used in combination. It is important to note that Staphylococci that are resistant to erythromycin may emerge during erythromycin and/or sulfonamide therapy. Pseudomembranous colitis has been reported with most antibacterial agents, including erythromycin, and may range in severity from mild to life-threatening. Therefore, the physician should consider this diagnosis in patients with diarrhea after the administration of antibacterial agents. Erythromycin can cause hepatic dysfunction, cholestatic jaundice, and abnormal liver transaminases, particularly when erythromycin estolate is administered.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
D10AF02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AF - Antiinfectives for treatment of acne
D10AF02 - Erythromycin
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA01 - Erythromycin
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AA - Antibiotics
S01AA17 - Erythromycin
Absorption
Orally administered erythromycin is readily absorbed. Food intake does not appear to exert effects on serum concentrations of erythromycin. Some interindividual variation exists in terms of erythromycin absorption, which may impact absorption to varying degrees. The Cmax of erythromycin is 1.8 mcg/L and the Tmax is 1.2 hours. The serum AUC of erythromycin after the administration of a 500mg oral dose was 7.33.9 mg.h/l in one pharmacokinetic study. Erythromycin is well known for a bioavailability that is variable (18-45%) after oral administration and its susceptibility to broken down under acidic conditions.
Route of Elimination
In patients with normal liver function, erythromycin concentrates in the liver and is then excreted in the bile.Under 5% of the orally administered dose of erythromycin is found excreted in the urine. A high percentage of absorbed erythromycin is not accounted for, but is likely metabolized.
Volume of Distribution
Erythromycin is found in most body fluids and accumulates in leucocytes and inflammatory liquid. Spinal fluid concentrations of erythromycin are low, however, the diffusion of erythromycin through the blood-brain barrier increases in meningitis, likely due to the presence of inflamed tissues which are easily penetrated. Erythromycin crosses the placenta.
Clearance
The clearance of erythromycin in healthy subjects was 0.53 0.13 l/h/kg after a 125mg intravenous dose. In a clinical study of healthy patients and patients with liver cirrhosis, clearance of erythromycin was significantly reduced in those with severe liver cirrhosis. The clearance in cirrhotic patients was 42.2 10.1 l h1 versus 113.2 44.2 l h-1 in healthy patients.
Absorption of orally administered erythromycins occurs mainly in the duodenum. The bioavailability of the drugs is variable and depends on several factors including the particular erythromycin derivative, the formulation of the dosage form administered, acid stability of the derivative, presence of food in the GI tract, and gastric emptying time.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 234
Erythromycin is rather slowly absorbed after oral administration. peak serum concentrations ranged from 0.1 to 4.8 ug/mL according to the form and the coating of erythromycin administered. The oral absorption is less that 50% and erythromycin is degraded by gastric acid. It is absorbed in the small intestine (mainly in duodenum for humans) as erythromycin base.
Erythromycin diffuses readily into intracellular fluids, achieving antibacterial activity in essentially all sites except the brain and CSF. Erythromycin penetrates into prostatic fluid, achieving concentrations approximately 40% of those in plasma. Concentrations in middle ear exudate reach only 50% of serum concentrations and thus may be inadequate for the treatment of otitis media caused by H. influenzae. Protein binding is approximately 70% to 80% for erythromycin base and even higher, 96%, for the estolate. Erythromycin traverses the placenta, and drug concentrations in fetal plasma are about 5% to 20% of those in the maternal circulation. Concentrations in breast milk are 50% of those in serum.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 1184
In an in vitro model using human skin, erythromycin was absorbed into the stratum corneum following topical application of 10-20 mg of the drug in a vehicle containing dimethylacetamide and 95% alcohol. The drug does not appear to be absorbed systemically following twice daily application of a 2% solution of the drug in a vehicle containing 77% alcohol and polyethylene glycol and acetone. It is not known if erythromycin is absorbed from intact or denuded skin, wounds, or mucous membranes following topical application of an ointment containing the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3512
For more Absorption, Distribution and Excretion (Complete) data for Erythromycin (13 total), please visit the HSDB record page.
Hepatic first-pass metabolism contributes significantly to erythromycin metabolism after an oral dose. Erythromycin is partially metabolized by CYP3A4 enzyme to N-desmethylerythromycin. Erythromycin is also hydrolyzed to _anhydro_ forms (anhydroerythromycin [AHE] and other metabolites), and this process is promoted by acidic conditions. AHE is inactive against microbes but inhibits hepatic drug oxidation and is therefore considered to be an important contributor to erythromycin drug-drug interactions.
Twenty hours after an oral administration of 10 mg erythromycin to rats, about 37-43% of the administered radioactivity was recovered in the intestinal tract plus feces, 27.2 to 36.1% in the urine, 21-29% in the expired air. It was rapidly metabolized in the liver, mainly through demethylation process, and excreted in the bile as des-N-methyl-erythromycin, the major metabolite present only in the bile and in the intestinal contents of rats. The isotropic methyl group was eliminated in the expired air as CO2.
The elimination half-life of oral erythromycin was 3.5 hours according to one study and ranged between 2.4-3.1 hours in another study. Repetitive dosing of erythromycin leads to increased elimination half-life.
... The serum elimination half-life of erythromycin is approximately 1.6 hours.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 1184
The serum half-life in normal subjects is 2 hours and in anuric subjects, 4-6 hours.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 360
In order to replicate, bacteria require a specific process of protein synthesis, enabled by ribosomal proteins. Erythromycin acts by inhibition of protein synthesis by binding to the 23S ribosomal RNA molecule in the 50S subunit of ribosomes in susceptible bacterial organisms. It stops bacterial protein synthesis by inhibiting the transpeptidation/translocation step of protein synthesis and by inhibiting the assembly of the 50S ribosomal subunit. This results in the control of various bacterial infections. The strong affinity of macrolides, including erythromycin, for bacterial ribosomes, supports their broadspectrum antibacterial activities.
Macrolide antibiotics are bacteriostatic agents that inhibit protein synthesis by binding reversibly to 50S ribosomal subunits of sensitive microorganisms, at or very near the site that binds chloramphenicol. Erythromycin does not inhibit peptide bond formation per se, but rather inhibits the translocation step wherein a newly synthesized peptidyl tRNA molecule moves from the acceptor site on the ribosome to the peptidyl donor site. Gram-positive bacteria accumulate about 100 times more erythromycin than do gram-negative bacteria. Cells are considerably more permeable to the un-ionized form of the drug, which probably explains the increased antimicrobial activity at alkaline pH.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 1183
... /Erythromycin/ inhibits the growth of susceptible organisms (principally Propionibacterium acnes) on the surface of the skin and reduces the concn of free fatty acids in sebum ... The reduction in free fatty acids in sebum may be an indirect result of the inhibition of lipase-producing organisms which convert triglycerides into free fatty acids or may be a direct result of interference with lipase production in these organisms. /In acne treatment regimens/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 2378
Although stromal-derived factor-1 (SDF-1) via its cognate receptor CXCR4 is assumed to play a critical role in migration of endothelial cells during new vessel formation after tissue injury, CXCR4 expression on endothelial cells is strictly regulated. Erythromycin (EM), a 14-membered ring macrolide, has an anti-inflammatory effect that may account for its clinical benefit in the treatment of chronic inflammatory diseases. However, the effects of EM on endothelial cells and especially their expression of CXCR4 have not been fully evaluated. In this study, we demonstrated that EM markedly induced CXCR4 surface expression on microvascular endothelial cells in vitro and lung capillary endothelial cells in vivo. This ability to induce CXCR4 surface expression on endothelial cells was restricted to 14-membered ring macrolides and was not observed in other antibiotics including a 16-membered ring macrolide, josamycin. Furthermore, this EM-induced expression of CXCR4 on endothelial cells was functionally significant as demonstrated by chemotaxis assays in vitro. These findings suggest that EM-induced CXCR4 surface expression on endothelial cells may promote migration of CXCR4-expressing endothelial cells into sites of tissue injury, which may be associated with the known anti-inflammatory activity of this macrolide.
PMID:19502290 Takagi Y et al; Am J Physiol Lung Cell Mol Physiol 297 (3): L420-31 (2009).

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Application : Disintegrants & Superdisintegrants
Excipient Details : Crospovidone is used as a disintegrant in solid oral dosage forms such as tablets and capsules.
Dosage Form : Capsule, Tablet, Topical Film, Transdermal Patch
Grade : Not Available
Category : Controlled & Modified Release, Direct Compression, Granulation
Application : Controlled & Modified Release, Direct Compression, Granulation
Excipient Details : For non-erodible matrices using direct compression, Controlled release matrix. Matrix former in transdermal patches and topical films.
Pharmacopoeia Ref : Ph. Eur., USP-NF, JP-JPE: 80 %...
Technical Specs : Not Available
Ingredient(s) : Lauryl Sulfate
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Fillers, Diluents & Binders, Granulation
Application : Direct Compression, Fillers, Diluents & Binders, Granulation
Excipient Details : AceCel is suitable for majority of the directly compressible actives, combines good flow and high compressibility.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Disintegrants & Superdisintegrants, Granulation
Application : Granulation
Excipient Details : Crospovidone is used as a granulating agent in pharmaceutical formulations such as tablets, granules, and pellets.
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Fillers, Diluents & Binders, Granulation
Application : Direct Compression, Fillers, Diluents & Binders, Granulation
Excipient Details : HiCel acts as a strong & dry binder. It facilitates low tablet friability & promotes rapid tablet disintegration.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Granule / Pellet, Tablet
Grade : Not Available
Category : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Granulation
Application : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Granulation
Excipient Details : Tablets, Granules, Pills, Disintegrants and fillers.
Application : Fillers, Diluents & Binders, Granulation
Grade : Not Available
Category : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Application : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Excipient Details : Controlled Release, Direct Compression,Wet Granulation,Tablet Coating, Liquid Solutions and Suspensions
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Tablet
Grade : Oral
Category : Coating Systems & Additives, Fillers, Diluents & Binders, Granulation
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?