
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
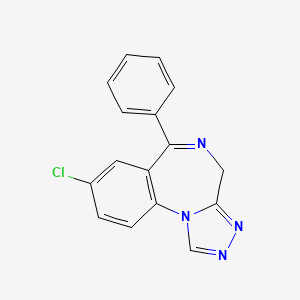

1. D-40ta
2. D40ta
3. Nuctalon
4. Prosom
5. Tasedan
1. 29975-16-4
2. Prosom
3. Eurodin
4. Nuctalon
5. Julodin
6. Esilgan
7. D 40ta
8. 8-chloro-6-phenyl-4h-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
9. Estazolamum
10. D-40ta
11. Abbott-47631
12. 8-chloro-6-phenyl-4h-s-triazolo(4,3-a)(1,4)benzodiazepine
13. Estazolam Civ
14. Nsc 290818
15. U 33737
16. Nsc-290818
17. 36s3eqv54c
18. Chebi:4858
19. 4h-(1,2,4)triazolo(4,3-a)(1,4)benzodiazepine, 8-chloro-6-phenyl-
20. 53180-72-6
21. Ncgc00168250-01
22. 8-chloro-6-phenyl-4h-2,3,5,10b-tetraaza-benzo[e]azulene
23. 8-chloro-6-phenyl-4h-s-triazolo[4,3-a][1,4]benzodiazepine
24. Nemurel
25. Somnatrol
26. Cannoc
27. 4h-s-triazolo(4,3-a)(1,4)benzodiazepine, 8-chloro-6-phenyl-
28. 4h-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine, 8-chloro-6-phenyl-
29. Abbott 47631
30. Estazolamum [inn-latin]
31. Estazolam [usan:inn:jan]
32. Prosom (tn)
33. Ccris 1955
34. Einecs 249-982-4
35. Brn 1220868
36. Unii-36s3eqv54c
37. 8-chloro-6-phenyl-4h-(1,2,4)triazolo(4,3-a)(1,4)benzodiazepine
38. Dea No. 2756
39. 8-chloro-6-phenyl-4h-(1,2,4)triazolo-(4,3-a)(1,4)benzodiazepine
40. 8-chloro-6-phenyl-4h-s-triazolo(4,3-a)(1,4)benzodiazepine (iupac)
41. Estazolam [inn]
42. Estazolam [jan]
43. Estazolam [mi]
44. Estazolam [iarc]
45. Estazolam [usan]
46. Dsstox_cid_572
47. Estazolam [vandf]
48. Estazolam [mart.]
49. Estazolam [who-dd]
50. Dsstox_rid_75665
51. Dsstox_gsid_20572
52. Schembl28766
53. Mls003899222
54. Bidd:gt0481
55. Estazolam (jp17/usp/inn)
56. Chembl285674
57. Gtpl7550
58. Zinc1370
59. Estazolam [orange Book]
60. Estazolam Civ [usp-rs]
61. Dtxsid5020572
62. Cdchdcwjmgxxrh-uhfffaoysa-
63. Estazolam [usp Monograph]
64. Estazolam 0.1 Mg/ml In Methanol
65. Estazolam 1.0 Mg/ml In Methanol
66. Bcp13317
67. Tox21_112609
68. Nsc290818
69. Akos005064373
70. Ab07556
71. Db01215
72. Smr000238168
73. Cas-29975-16-4
74. Wln: T B576 Bn Dnn Hn Ghj Ir& Lg
75. C06981
76. D00311
77. 975e164
78. A820132
79. Q-201071
80. Q3045264
81. 8-chloro-6-phenyl-4h-s-triazolo[4,4]benzodiazepine
82. 4h-s-triazolo[4,4]benzodiazepine, 8-chloro-6-phenyl-
83. Estazolam Solution, Drug Standard, 100 Mug/l In Methanol
84. 8-chloro-6-phenyl-4h-s-triazolo [4,3-a][1,4] Benzodiazepine
85. 8-chloro-6-phenyl-4h-s-triazolo-[4,3-a][1,4]benzodiazepine
86. Estazolam, United States Pharmacopeia (usp) Reference Standard
87. 4h-[1,4]triazolo[4,3-a][1,4]benzodiazepine, 8-chloro-6-phenyl-
88. 8-chloro-6-phenyl-4h-[1,4]triazolo[4,3-a][1,4]benzodiazepine
89. 8-chloro-6-phenyl-4h-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepine
90. Estazolam Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
91. 12-chloro-9-phenyl-2,4,5,8-tetraazatricyclo[8.4.0.0?,?]tetradeca-1(14),3,5,8,10,12-hexaene
92. 12-chloro-9-phenyl-2,4,5,8-tetraazatricyclo[8.4.0.0^{2,6}]tetradeca-1(10),3,5,8,11,13-hexaene
| Molecular Weight | 294.74 g/mol |
|---|---|
| Molecular Formula | C16H11ClN4 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 294.0672241 g/mol |
| Monoisotopic Mass | 294.0672241 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 407 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Estazolam |
| PubMed Health | Estazolam (By mouth) |
| Drug Classes | Hypnotic |
| Drug Label | Estazolam, a triazolobenzodiazepine derivative, is an oral hypnotic agent. Estazolam occurs as a fine, white, odorless powder that is soluble in alcohol and practically insoluble in water. The chemical name for estazolam is 8-chloro-6-phenyl-4H-s-tri... |
| Active Ingredient | Estazolam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2mg; 1mg |
| Market Status | Prescription |
| Company | Watson Labs; Teva; Par Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Estazolam |
| PubMed Health | Estazolam (By mouth) |
| Drug Classes | Hypnotic |
| Drug Label | Estazolam, a triazolobenzodiazepine derivative, is an oral hypnotic agent. Estazolam occurs as a fine, white, odorless powder that is soluble in alcohol and practically insoluble in water. The chemical name for estazolam is 8-chloro-6-phenyl-4H-s-tri... |
| Active Ingredient | Estazolam |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2mg; 1mg |
| Market Status | Prescription |
| Company | Watson Labs; Teva; Par Pharm |
For the short-term management of insomnia characterized by difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakenings.
Estazolam, a triazolobenzodiazepine derivative, is an oral hypnotic agent with anticonvulsant, hypnotic, and muscle relaxant properties. It has been shown in some cases to be more potent than diazepam or nitrazepam.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CD - Benzodiazepine derivatives
N05CD04 - Estazolam
Absorption
Tablets have been found to be equivalent in absorption to an orally administered solution of estazolam. In healthy subjects who received up to three times the recommended dose, peak estazolam plasma concentrations occurred within two hours after dosing (range 0.5 to 6.0 hours) and were proportional to the administered dose, suggesting linear pharmacokinetics over the dosage range tested.
Route of Elimination
Estazolam is extensively metabolized. The elimination of the parent drug takes place via hepatic metabolism of estazolam to hydroxylated and other metabolites that are eliminated largely in the urine both free and conjugated. Less than 5% of a 2 mg dose of estazolam was excreted unchanged in the urine, with only 4% of the dose appearing in the feces. Radiolabel mass balance studies indicate that the main route of excretion is via the kidneys. After 5 days, 87% of the administered radioactivity was excreted in human urine. Less than 4% of the dose was excreted unchanged.
Extensively metabolized in the liver. In vitro studies with human liver microsomes indicate that the biotransformation of estazolam to the major circulating metabolite 4-hydroxy-estazolam is mediated by cytochrome P450 3A (CYP3A).
Estazolam has known human metabolites that include 4-hydroxyestazolam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The range of estimates for the mean elimination half-life of estazolam varies from 10 to 24 hours.
Benzodiazepines bind nonspecifically to benzodiazepine receptors, which affects affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects GABA by increasing GABA affinity for the GABA receptor. Binding of the inhibitory neurotransmitter GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
82
PharmaCompass offers a list of Estazolam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Estazolam manufacturer or Estazolam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Estazolam manufacturer or Estazolam supplier.
PharmaCompass also assists you with knowing the Estazolam API Price utilized in the formulation of products. Estazolam API Price is not always fixed or binding as the Estazolam Price is obtained through a variety of data sources. The Estazolam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Esilgan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Esilgan, including repackagers and relabelers. The FDA regulates Esilgan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Esilgan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Esilgan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Esilgan supplier is an individual or a company that provides Esilgan active pharmaceutical ingredient (API) or Esilgan finished formulations upon request. The Esilgan suppliers may include Esilgan API manufacturers, exporters, distributors and traders.
click here to find a list of Esilgan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Esilgan DMF (Drug Master File) is a document detailing the whole manufacturing process of Esilgan active pharmaceutical ingredient (API) in detail. Different forms of Esilgan DMFs exist exist since differing nations have different regulations, such as Esilgan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Esilgan DMF submitted to regulatory agencies in the US is known as a USDMF. Esilgan USDMF includes data on Esilgan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Esilgan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Esilgan suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Esilgan Drug Master File in Japan (Esilgan JDMF) empowers Esilgan API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Esilgan JDMF during the approval evaluation for pharmaceutical products. At the time of Esilgan JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Esilgan suppliers with JDMF on PharmaCompass.
A Esilgan written confirmation (Esilgan WC) is an official document issued by a regulatory agency to a Esilgan manufacturer, verifying that the manufacturing facility of a Esilgan active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Esilgan APIs or Esilgan finished pharmaceutical products to another nation, regulatory agencies frequently require a Esilgan WC (written confirmation) as part of the regulatory process.
click here to find a list of Esilgan suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Esilgan as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Esilgan API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Esilgan as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Esilgan and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Esilgan NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Esilgan suppliers with NDC on PharmaCompass.
Esilgan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Esilgan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Esilgan GMP manufacturer or Esilgan GMP API supplier for your needs.
A Esilgan CoA (Certificate of Analysis) is a formal document that attests to Esilgan's compliance with Esilgan specifications and serves as a tool for batch-level quality control.
Esilgan CoA mostly includes findings from lab analyses of a specific batch. For each Esilgan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Esilgan may be tested according to a variety of international standards, such as European Pharmacopoeia (Esilgan EP), Esilgan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Esilgan USP).
