
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
FDA Orange Book

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
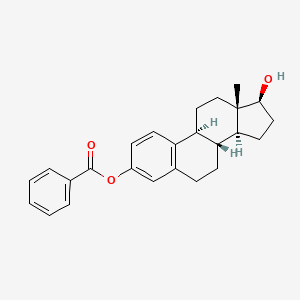

1. 17beta-estradiol-3-benzoate
2. Eston-b
3. Estradiol 3-benzoate
4. Oestradiol 3-benzoate
1. 50-50-0
2. Benzhormovarine
3. Benzoestrofol
4. Benovocylin
5. Estradiol 3-benzoate
6. Oestradiol Benzoate
7. Estradiol Monobenzoate
8. Benzofoline
9. Follidrin
10. Ovasterol-b
11. Benzo-gynoestryl
12. Primogyn B
13. Progynon B
14. Progynon Benzoate
15. Eston-b
16. Diffollisterol
17. Difolliculine
18. Femestrone
19. Follicormon
20. Gynecormone
21. Gynformone
22. Hidroestron
23. Hormogynon
24. Unistradiol
25. Benztrone
26. Graafina
27. Solestro
28. Dimenformon Benzoate
29. De Graafina
30. Ovocyclin M
31. Primogyn I
32. Ovocyclin-mb
33. Diogyn B
34. Oestradiol 3-benzoate
35. Oestradiol Monobenzoate
36. Ovahormon Benzoate
37. Ovocyclin Benzoate
38. Agofollin Depot
39. 17-beta-estradiol 3-benzoate
40. Beta-estradiol 3-benzoate
41. Recthormone Oestradiol
42. Benzoic Acid Estradiol
43. Estradioli Benzoas
44. Benzoate D'estradiol
45. Benzoate D'oestradiol
46. Benzoato De Estradiol
47. 17beta-estradiol 3-benzoate
48. 17beta-estradiol Monobenzoate
49. Estradiol, 3-benzoate
50. Benzestrofol
51. Benzogynestryl
52. Dimenformone
53. Estrogin
54. Mesalin
55. Folone
56. Nsc-9566
57. Oestroform [bdh]
58. Dihydroestrin Benzoate
59. Hydroxyestrin Benzoate
60. [(8r,9s,13s,14s,17s)-17-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl] Benzoate
61. Benzoestrofol Difolliculin
62. Dihydrofolliculin Benzoate
63. .beta.-estradiol Benzoate
64. 1s4cjb5zgn
65. Estradiol (benzoate)
66. .beta.-estradiol 3-benzoate
67. 17.beta.-estradiol Benzoate
68. Estradiol-17.beta. Benzoate
69. Mls000028477
70. Ebz
71. 17.beta.-estradiol 3-benzoate
72. Estradiol-17.beta. 3-benzoate
73. 17.beta.-estradiol Monobenzoate
74. Chebi:77006
75. Ostrin
76. Ncgc00021274-03
77. (17beta)-17-hydroxyestra-1(10),2,4-trien-3-yl Benzoate
78. Metroval
79. Reglovar
80. Smr000058343
81. Benzo-ginestryl
82. Estradiolo Amsa
83. Benzo-gineostril
84. Oestradioli Benzoas
85. Gynecormone Gouttes
86. Primogyn B Oleosum
87. (8r,9s,13s,14s,17s)-17-hydroxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthren-3-yl Benzoate
88. Oestraform (bdh)
89. Oestradiolum Benzoicum
90. Oestradiolum Benzoylatum
91. (17beta)-estra-1,3,5(10)-triene-3,17-diol 3-benzoate
92. Dihydrofolliculine Benzoate
93. Estradiol Benzoate (van)
94. Estradiol Benzoate [inn]
95. 17-beta-estradiol Benzoate
96. Estradiolo Benzoato
97. Estradiolo Benzoato [dcit]
98. Estradiol-17beta 3-benzoate
99. Ccris 281
100. Estradiol-17-beta-3-benzoate
101. Benzoate D'oestradiol [french]
102. 17-beta-oestradiol 3-benzoate
103. Estradioli Benzoas [inn-latin]
104. Benzoate D'estradiol [inn-french]
105. Nsc 9566
106. Einecs 200-043-7
107. Unii-1s4cjb5zgn
108. Benzoato De Estradiol [inn-spanish]
109. Brn 3107526
110. Benzogynoestryl
111. Difolliculin
112. Oestraform
113. Ai3-52465
114. Pelanin Benzoate
115. Progynon-b
116. Primogyn Boleosum
117. Estradiol-benzoate
118. Diffolisterol,(s)
119. Estra-1,3,5(10)-triene-3,17beta-diol, 3-benzoate
120. Estra-1,3,5(10)-triene-3,17-beta-diol, 3-benzoate
121. Estradiol Benzoate [usp:inn:ban:jan]
122. Estra-1,3,5(10)-triene-3,17-diol (17-beta)-3-benzoate
123. St075190
124. Ss-estradiol 3-benzoate
125. 17-estradiol 3-benzoate
126. Dsstox_cid_2998
127. .beta.-oestradiol Benzoate
128. 3-benzoyloxy-17ss-estrol
129. Dsstox_rid_76823
130. Dsstox_gsid_22998
131. I(2)-estradiol 3-benzoate
132. 4-09-00-00406 (beilstein Handbook Reference)
133. Mls002207215
134. .beta.-oestradiol 3-benzoate
135. Bidd:er0126
136. Schembl174896
137. Chembl282575
138. Cid_222757
139. Dtxsid9022998
140. Estradiol Benzoate [jan]
141. Bdbm56905
142. Estradiol Benzoate (jp17/usp)
143. Nsc9566
144. 17-.beta.-oestradiol 3-benzoate
145. Hms2232p14
146. Estradiol 3-benzoate [mi]
147. Estradiol Benzoate [mart.]
148. Amy22166
149. Bcp09252
150. Estra-1,3,5(10)-triene-3,17-diol, (17beta)-, 3-benzoate
151. Estradiol Benzoate [usp-rs]
152. Estradiol Benzoate [who-dd]
153. Hy-b1192
154. Zinc3881345
155. Tox21_110868
156. Beta-estradiol 3-benzoate, >=97%
157. S4110
158. Akos015955542
159. Ccg-268370
160. Cs-4780
161. Db13953
162. Estradiol Benzoate [green Book]
163. Cas-50-50-0
164. Estradiol Benzoate [ep Monograph]
165. Estradiol Benzoate [usp Impurity]
166. Estradiol Benzoate For System Suitability
167. Ncgc00021274-04
168. Ncgc00021274-05
169. As-13030
170. Estradiol Benzoate [usp Monograph]
171. Wln: L E5 B666ttt&j E1 Fq Oovr
172. E0329
173. D01953
174. D97619
175. 003e692
176. A828140
177. Sr-01000003080
178. 1,5(10)-estratriene-3,17.beta.-diol 3-benzoate
179. Q-201505
180. Sr-01000003080-3
181. 1,3,5(10)-estratriene-3,17.beta.-diol 3-benzoate
182. 3,17ss-dihydroxy-1,3,5(10)-estratriene 3-benzoate
183. Estra-1,3,5(10)-triene-3,17beta-diol 3-benzoate
184. Estra-1,5(10)-triene-3,17.beta.-diol, 3-benzoate
185. Q11450699
186. (17beta)estra-1,3,5(10)triene-3,17-diol-3-benzoate
187. 1,3,5(10)-oestratriene-3,17-.beta.-diol 3-benzoate
188. 3-benzoyloxy-17.beta.-hydroxyestra-1,3,5(10)-triene
189. Estra-1,3,5(10)-triene-3,17.beta.-diol, 3-benzoate
190. (17?)-17-hydroxyestra-1(10),2,4-trien-3-yl Benzoate
191. Estra-1,3,5(10)-triene-3,17-diol (17b)-, 3-benzoate
192. (17.beta.)-estra-1,3,5(10)-triene-3,17-diol 3-benzoate
193. Beta-estradiol 3-benzoate, Vetranal(tm), Analytical Standard
194. Estra-1,5(10)-triene-3,17-diol (17.beta.)-, 3-benzoate
195. 17-hydroxyestra-1(10),2,4-trien-3-yl Benzoate, (17.beta.)-
196. Estradiol Benzoate, European Pharmacopoeia (ep) Reference Standard
197. Estradiol Benzoate, United States Pharmacopeia (usp) Reference Standard
198. Estradiol Benzoate For System Suitability, European Pharmacopoeia (ep) Reference Standard
199. (8r,9s,13s,14s,17s)-17-hydroxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthren-3-yl Benzoate;17beta-estradiol Benzoate; 1,3,5(10)-estratriene-3,17b-diol 3-benzoate; 3,17b-dihydroxy-1,3,5(10)-estratriene 3-benzoate
200. [(13s,17s)-17-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl] Benzoate
201. [(8r,9s,13s,14s,17s)-13-methyl-17-oxidanyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl] Benzoate
202. Benzoic Acid [(8r,9s,13s,14s,17s)-17-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl] Ester
| Molecular Weight | 376.5 g/mol |
|---|---|
| Molecular Formula | C25H28O3 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 376.20384475 g/mol |
| Monoisotopic Mass | 376.20384475 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 582 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Estradiol benzoate is not currently available in any FDA or Health Canada approved products.
Estradiol, the principal intracellular human estrogen, is substantially more active than its metabolites, estrone and estriol, at the cellular level.
Contraceptive Agents, Hormonal
Contraceptive agents that act on the ENDOCRINE SYSTEM. (See all compounds classified as Contraceptive Agents, Hormonal.)
Exogenous estrogens are metabolized using the same mechanism as endogenous estrogens. Estrogens are partially metabolized by cytochrome P450.
Estradiol enters target cells freely (e.g., female organs, breasts, hypothalamus, pituitary) and interacts with a target cell receptor. When the estrogen receptor has bound its ligand it can enter the nucleus of the target cell, and regulate gene transcription which leads to formation of messenger RNA. The mRNA interacts with ribosomes to produce specific proteins that express the effect of estradiol upon the target cell. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
78
PharmaCompass offers a list of Estradiol Benzoate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Estradiol Benzoate manufacturer or Estradiol Benzoate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Estradiol Benzoate manufacturer or Estradiol Benzoate supplier.
PharmaCompass also assists you with knowing the Estradiol Benzoate API Price utilized in the formulation of products. Estradiol Benzoate API Price is not always fixed or binding as the Estradiol Benzoate Price is obtained through a variety of data sources. The Estradiol Benzoate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Estradiol Benzoate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Estradiol Benzoate, including repackagers and relabelers. The FDA regulates Estradiol Benzoate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Estradiol Benzoate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Estradiol Benzoate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Estradiol Benzoate supplier is an individual or a company that provides Estradiol Benzoate active pharmaceutical ingredient (API) or Estradiol Benzoate finished formulations upon request. The Estradiol Benzoate suppliers may include Estradiol Benzoate API manufacturers, exporters, distributors and traders.
click here to find a list of Estradiol Benzoate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Estradiol Benzoate DMF (Drug Master File) is a document detailing the whole manufacturing process of Estradiol Benzoate active pharmaceutical ingredient (API) in detail. Different forms of Estradiol Benzoate DMFs exist exist since differing nations have different regulations, such as Estradiol Benzoate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Estradiol Benzoate DMF submitted to regulatory agencies in the US is known as a USDMF. Estradiol Benzoate USDMF includes data on Estradiol Benzoate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Estradiol Benzoate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Estradiol Benzoate suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Estradiol Benzoate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Estradiol Benzoate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Estradiol Benzoate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Estradiol Benzoate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Estradiol Benzoate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Estradiol Benzoate suppliers with NDC on PharmaCompass.
Estradiol Benzoate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Estradiol Benzoate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Estradiol Benzoate GMP manufacturer or Estradiol Benzoate GMP API supplier for your needs.
A Estradiol Benzoate CoA (Certificate of Analysis) is a formal document that attests to Estradiol Benzoate's compliance with Estradiol Benzoate specifications and serves as a tool for batch-level quality control.
Estradiol Benzoate CoA mostly includes findings from lab analyses of a specific batch. For each Estradiol Benzoate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Estradiol Benzoate may be tested according to a variety of international standards, such as European Pharmacopoeia (Estradiol Benzoate EP), Estradiol Benzoate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Estradiol Benzoate USP).

