
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book
0
Canada
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
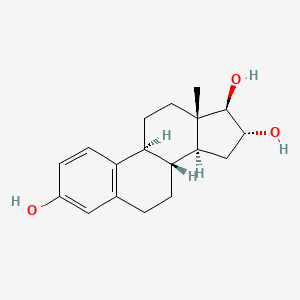

1. (16alpha,17beta)-estra-1,3,5(10)-triene-3,16,17-triol
2. (16beta,17beta)-estra-1,3,5(10)-triene-3,16,17-triol
3. 16 Alpha Hydroxy Estradiol
4. 16-alpha-hydroxy-estradiol
5. 16alpha,17beta Estriol
6. 16alpha,17beta-estriol
7. 16beta Hydroxy Estradiol
8. 16beta-hydroxy-estradiol
9. Epiestriol
10. Estra-1,3,5(10)-triene-3,16beta,17beta-triol
11. Ovestin
1. 50-27-1
2. Oestriol
3. Trihydroxyestrin
4. Aacifemine
5. Estratriol
6. Ovestin
7. Ovestrion
8. Destriol
9. Tridestrin
10. Theelol
11. Oestratriol
12. Orestin
13. Holin
14. 16alpha-hydroxyestradiol
15. Ortho-gynest
16. Deuslon-a
17. Hemostyptanon
18. Hormomed
19. Klimoral
20. Oestriolum
21. Orgastyptin
22. Overstin
23. Thulol
24. Triovex
25. 16alpha,17beta-estriol
26. Gynaesan
27. Hormonin
28. Stiptanon
29. Synapause
30. Triodurin
31. Estriel
32. Trihydroxyoestrin
33. Follicular Hormone Hydrate
34. 16-alpha-hydroxyestradiol
35. Estriolo [italian]
36. 16alpha-hydroxyoestradiol
37. 16alpha,17beta-oestriol
38. 16-alpha,17-beta-estriol
39. 16-alpha,17-beta-oestriol
40. Colpogyn
41. Estriolo
42. Incurin
43. 16alpha-hydroxy-17beta-estradiol
44. Estriol, Unconjugated
45. (16alpha,17beta)-estra-1,3,5(10)-triene-3,16,17-triol
46. 16-alpha-hydroxyoestradiol
47. Deuslon A
48. Nsc-12169
49. 3,16alpha,17beta-estriol
50. 3,16-alpha,17-beta-estriol
51. 3,16-alpha,17-beta-oestriol
52. 16.alpha.-hydroxyestradiol
53. 16.alpha.,17.beta.-estriol
54. 3,16alpha,17beta-trihydroxy-1,3,5(10)-estratriene
55. 16.alpha.-estriol
56. Chlorapatite
57. Gynasan
58. Ovesterin
59. 1,3,5-estratriene-3beta,16alpha,17beta-triol
60. Ovo-vinces
61. 1,3,5-oestratriene-3beta,16alpha,17beta-triol
62. A 13610
63. Klimax E
64. Estra-1,3,5(10)-trien-3,16alpha,17beta-triol
65. Oestra-1,3,5(10)-triene-3,16alpha,17beta-triol
66. 16.alpha.-hydroxyoestradiol
67. 3,16alpha,17beta-trihydroxy-delta-1,3,5-oestratriene
68. 3,16-alpha,17-beta-trihydroxy-delta-1,3,5-estratriene
69. 3,16-alpha,17-beta-trihydroxy-delta-1,3,5-oestratriene
70. 3,16-alpha,17-beta-trihydroxyestra-1,3,5(10)-triene
71. 3,16-alpha,17-beta-trihydroxyoestra-1,3,5(10)-triene
72. (16alpha,17beta)-oestra-1,3,5(10)-triene-3,16,17-triol
73. 1,3,5(10)-estratriene-3,16alpha,17beta-triol
74. 16.alpha.,17.beta.-oestriol
75. 1,3,5(10)-estratriene-3,16-alpha,17beta-triol
76. Chebi:27974
77. Estra-1,3,5(10)-triene-3,16alpha,17beta-triol
78. Oestra-1,3,5(10)-triene-3,16-alpha,17-beta-triol
79. 3,16alpha,17beta-trihydroxy-delta(1,3,5)-estratriene
80. (8r,9s,13s,14s,16r,17r)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,16,17-triol
81. Oe3
82. 16.alpha.-hydroxy-17.beta.-estradiol
83. 16-epiestriol
84. Fb33469r8e
85. Nsc12169
86. Estra-1,3,5(10)-triene-3,16,17-triol, (16a,17b)-
87. Estra-1,3,5(10)-triene-3,16,17-triol
88. Dsstox_cid_2366
89. Dsstox_rid_76559
90. Dsstox_gsid_22366
91. 1306-04-3
92. 3,16.alpha.,17.beta.-estriol
93. Mfcd00003691
94. 16a-hydroxyestradiol
95. Folicular Hormone
96. (1s,10r,11s,13r,14r,15s)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-5,13,14-triol
97. 3,17.beta.-estriol
98. Ccris 284
99. Estriel (tn)
100. 16-hydroxyestradiol
101. Oestriol [steroidal Oestrogens]
102. Estra-1,3,5(10)-triene-3,16,17-triol, (16.alpha.,17.beta.)-
103. Hsdb 3590
104. Sr-01000721851
105. Einecs 200-022-2
106. Nsc 12169
107. 1,3,5(10)-estratriene-3,16.alpha.,17.beta.-triol
108. Estra-1,3,5(10)-trien-3,16.alpha.,17.beta.-triol
109. Estra-1,3,5(10)-triene-3,16.alpha.,17.beta.-triol
110. 1,3,5(10)-estratriene-3,16,17-triol
111. 1,3,5-estratriene-3.beta.,16-.alpha.,17-.beta.-triol
112. 1,3,5-oestratriene-3-.beta.,16.alpha.,17.beta.-triol
113. 3,16.alpha.,17.beta.-trihydroxy-1,3,5(10)-estratriene
114. 3,16.alpha.,17.beta.-trihydroxyestra-1,3,5(10)-triene
115. Brn 2508172
116. Oestra-1,3,5(10)-triene-3,16.alpha., 17.beta.-triol
117. 3,16.alpha.,17.beta.-trihydroxy-.delta.-1,3,5-estratriene
118. Ovestinon
119. Trimesta
120. 3,16.alpha.,17.beta.-trihydroxy-.delta.-1,3,5-oestratriene
121. Oestriol.
122. Unii-fb33469r8e
123. (16.alpha.,17.beta.)-estra-1,3,5(10)-triene-3,16,17-triol
124. (16.alpha.,17.beta.)-oestra-1,3,5(10)-triene-3,16,17-triol
125. Estriol [usp:inn:ban:jan]
126. 3,16-.alpha.,17-.beta.-oestriol
127. Estra-1,3,5(10)-triene-3,16,17-triol, (16alpha,17beta)-
128. Holin V
129. Cas-50-27-1
130. 16a-estriol
131. Ncgc00166111-01
132. (8r,9s,13s,14s,16r,17r)-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,16,17-triol
133. 16a,17b-estriol
134. Estriol, 98%
135. Estriol [hsdb]
136. Estriol, >=97%
137. Estriol [jan]
138. Estriol [mi]
139. 3,16a,17b-estriol
140. Estriol [mart.]
141. Prestwick0_001096
142. Prestwick1_001096
143. Prestwick2_001096
144. Prestwick3_001096
145. Estriol (jp17/usp)
146. E0218
147. Estriol [usp-rs]
148. Estriol [who-dd]
149. Epitope Id:140131
150. 1,3,5(10)-estratrien-3,16.alpha.,17.beta.-triol
151. Estra-1,3,5(10)-triene-3,16-.alpha., L7-.beta.-triol
152. Schembl78033
153. Bspbio_001172
154. Estriol [green Book]
155. 3,16-.alpha.,17-.beta.-trihydroxyoestra-1,3,5(10)-triene
156. 4-06-00-07550 (beilstein Handbook Reference)
157. Mls000069812
158. Bidd:er0124
159. Spbio_003056
160. Estriol [ep Monograph]
161. Bpbio1_001290
162. Chembl193482
163. Gtpl2821
164. Estriol [usp Monograph]
165. Dtxsid9022366
166. Estriol 1.0 Mg/ml In Methanol
167. Hms1571k14
168. Hms2090e20
169. Hms2098k14
170. Hms2234a18
171. Hms3715k14
172. Hms3884p19
173. Bcp23357
174. Hy-b0412
175. Zinc3815418
176. Estriol 100 Microg/ml In Methanol
177. Tox21_112320
178. Tox21_301604
179. Bdbm50410506
180. Lmst02010003
181. S2466
182. Estriol [ema Epar Veterinary]
183. Estriol 1000 Microg/ml In Methanol
184. Akos015894926
185. Tox21_112320_1
186. Bcp9000661
187. Ccg-221096
188. Db04573
189. Estriol 100 Microg/ml In Acetonitrile
190. Estriol, Purum, >=97.0% (hplc)
191. Fd12050
192. Smp1_000122
193. Ncgc00166111-02
194. Ncgc00179277-01
195. Ncgc00255193-01
196. Ncgc00274080-01
197. As-13735
198. Estriol, Meets Usp Testing Specifications
199. Smr000059210
200. Bcp0726000219
201. Ab00514045
202. B1507
203. Estriol, Vetranal(tm), Analytical Standard
204. Wln: L E5 B666ttt&j E1 Fq Gq Oq
205. C05141
206. D00185
207. Estra-1,3,5(10)-triene-3,16a,17b-triol
208. 1,3,5-estratriene-3b,16a,17b-triol
209. 3,16a,17b-trihydroxyestra-1,3,5(10)-triene
210. 3,17.beta.-trihydroxy-1,3,5(10)-estratriene
211. 3,17.beta.-trihydroxyestra-1,3,5(10)-triene
212. Ab00514045-08
213. Ab00514045-09
214. Ab00514045_10
215. (16.alpha.,3,5(10)-triene-3,16,17-triol
216. 003e691
217. Q409721
218. 3,17.beta.-trihydroxy-.delta.-1,3,5-estratriene
219. 3,17.beta.-trihydroxy-.delta.-1,3,5-oestratriene
220. Q-201072
221. Sr-01000721851-3
222. Sr-01000721851-4
223. 1,5(10)-estratriene-3,16.alpha., 17.beta.-triol
224. 1,5(10)-estratriene-3,16.alpha.,17.beta.-triol
225. 3,16alpha,17beta-trihydroxyestra-1,3,5(10)-triene
226. Brd-k17016787-001-03-5
227. Brd-k17016787-001-16-7
228. Estra-1,5(10)-trien-3,16.alpha.,17.beta.-triol
229. Estra-1,5(10)-triene-3,16.alpha.,17.beta.-triol
230. 1,5-estratriene-3.beta.,16-.alpha.,17-.beta.-triol
231. 1,5-oestratriene-3-.beta.,16.alpha.,17.beta.-triol
232. 13b-methyl-1,3,5(10)-gonatriene-3,16a,17b-triol
233. Estra-1,5(10)-trien-3,16.alpha., 17.beta.-triol
234. Estra-1,5(10)-triene-3,16.alpha., 17.beta.-triol
235. Estriol, European Pharmacopoeia (ep) Reference Standard
236. Oestra-1,5(10)-triene-3,16.alpha., 17.beta.-triol
237. Oestra-1,5(10)-triene-3,16.alpha.,17.beta.-triol
238. 6ec6f23b-a991-4606-8bc7-146d915dab31
239. Estriol, United States Pharmacopeia (usp) Reference Standard
240. (16a,17b)-estra-1,3,5(10)-triene-3,16,17-triol
241. (s)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acidt-butyl Ester
242. Estra-1(10),2,4-triene-3,16,17-triol, (16alpha,17beta)-
243. Estra-1,5(10)-triene-3,16,17-triol, (16.alpha.,17.beta.)-
244. (9beta,13alpha,16beta,17beta)-estra-1(10),2,4-triene-3,16,17-triol
245. 13.beta.-methyl-1,3,5(10)-gonatriene-3,16.alpha.,17.beta.-triol
246. Estriol For System Suitability, European Pharmacopoeia (ep) Reference Standard
247. Estriol Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
248. (13s,16r,17r)-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,16,17-triol
249. (8r,13s,16r,17r)-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,16,17-triol
250. (8r,9s,13s,14s,16r,17r)-13-methyl-6,7,8,9,11,12,14,15,16, 17-decahydrocyclopenta[a]phenanthrene-3,16,17-triol
| Molecular Weight | 288.4 g/mol |
|---|---|
| Molecular Formula | C18H24O3 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 288.17254462 g/mol |
| Monoisotopic Mass | 288.17254462 g/mol |
| Topological Polar Surface Area | 60.7 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 411 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/Estriol is indicated as/ hormone replacement therapy for treatment of atrophic vaginitis and kraurosis in post-menopausal women.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
/Estriol is indicated for the/ treatment of pruritus vulvae and dyspareunia associated with atrophic vaginal epithelium.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
Gynest Cream is not indicated during pregnancy. If pregnancy occurs during use of Gynest Cream, treatment should be withdrawn immediately.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
Gynest Cream contains arachis oil (peanut oil) and should not be applied by patients known to be allergic to peanuts. As there is a possible relationship between allergy to peanuts and allergy to soya, patients with soya allergy should also avoid Gynest Cream.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
Before initiating or re-instituting HRT, a complete personal and family medical history should be taken. Physical (including pelvic and breast) examination should be guided by this and by the contra-indications and warnings for use. During treatment, periodic check-ups are recommended of a frequency and nature adapted to the individual woman. Women should be advised what changes in their breasts should be reported to their doctor or nurse. Investigations, including mammography, should be carried out in accordance with currently accepted screening practices, modified to the clinical needs of the individual.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
The risk of endometrial hyperplasia and carcinoma is increased when systemic estrogens are administered alone for prolonged periods of time. The endometrial safety of long-term or repeated use of topical vaginal estrogens is uncertain. Therefore, if repeated, treatment should be reviewed at least annually, with a special consideration given to any symptoms of endometrial hyperplasia or carcinoma.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
For more Drug Warnings (Complete) data for ESTRIOL (40 total), please visit the HSDB record page.
Used as a test to determine the general health of an unborn fetus.
The treatment of hormone-dependent urinary incontinence due to sphincter mechanism incompetence in ovariohysterectomised bitches.
Estriol (also oestriol) is one of the three main estrogens produced by the human body. It is only produced in significant amounts during pregnancy as it is made by the placenta. In pregnant women with multiple sclerosis (MS), estriol reduces the disease's symptoms noticeably, according to researchers at UCLA's Geffen Medical School.
QG03CA04
G03CA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03C - Estrogens
G03CA - Natural and semisynthetic estrogens, plain
G03CA04 - Estriol
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03C - Estrogens
G03CC - Estrogens, combinations with other drugs
G03CC06 - Estriol
Estriol is readily absorbed following intravaginal application. Peak serum estriol concentrations are generally observed within 2 hours following intravaginal application and remain elevated for 6 hours.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
Systemic bioavailability on vaginal administration is better than after oral administration. Intravaginal application of 1 mg estriol in women with senile atrophy of the vaginal epithelium results in serum levels similar to those seen after oral administration of 10 mg estriol.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
Plasma estriol levels increased from <90pmol/L (26 pg/mL) about fifty fold over a few hours after intravaginal administration of Gynest Cream. Eight to ten hours after administration, 50% of women still had estriol levels above 90pmol/L (26 pg/mL).
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
Estriol circulates with the blood, about 14% free, 8% bound to SHBG and the rest bound to albumin.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
More than 95% of estriol is excreted in the urine, predominantly in the form of glucuronides.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
Primary metabolites of estriol include the 16-alpha-glucuronide, 3-glucuronide, 3-sulfate and 3-sulfate 16-alpha-glucuronide.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
The metabolic disposition of estrogens includes oxidative metabolism (largely hydroxylation) and conjugative metabolism by glucuronidation, sulfonation and/or O-methylation. Estradiol is converted to estrone by a 17beta-hydroxysteroid dehydrogenase; the estrone produced is further metabolized to 16alpha-hydroxyoestrone and then to estriol.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 145 (2007)
Estriol is a common metabolite of estrone and estradiol-17-beta in animals and in humans. Estriol is excreted in humans as conjugated and unconjugated 2-hydroxy estriol after 2-hydroxylation.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V21 336 (1979)
Estriol levels can be measured to give an indication of the general health of the fetus. DHEA-S is produced by the adrenal cortex of the fetus. This is converted to estriol by the placenta. If levels of "unconjugated estriol" are abnormally low in a pregnant woman, this may indicate a problem with the development of the child. The drug interacts with a target cell receptor. When the estrogen receptor has bound its ligand it can enter the nucleus of the target cell, and regulate gene transcription which leads to formation of messenger RNA. The mRNA interacts with ribosomes to produce specific proteins that express the effect of estriol upon the target cell. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary.
... Synthetic estriol, is chemically and biologically identical to endogenous human estriol. Estriol, a weak estrogen, is a natural metabolite of estradiol, the predominant estrogen. Estriol exerts estrogenicity by binding to estrogen receptors, present in the female genital tract. Estriol, oral or vaginal, similar to estradiol, corrects lowered proliferation and abnormal physiology in the atrophic vaginal epithelium seen in estrogen deficient states, such as after natural or surgical menopause. In contrast, the histology of the endometrium after using Gynest Cream rarely shows minor signs of proliferation in previously atrophic endometria.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Gynest 0.01% w/w Cream (Last updated June 2009). Available from, as of March 16, 2011: https://www.medicines.org.uk/EMC/medicine/24108/SPC/Gynest+0.01++w+w+Cream/
Estrogens have an important role in the reproductive, skeletal, cardiovascular, and central nervous systems in women, and act principally by regulating gene expression. Biologic response is initiated when estrogen binds to a ligand-binding domain of the estrogen receptor resulting in a conformational change that leads to gene transcription through specific estrogen response elements (ERE) of target gene promoters; subsequent activation or repression of the target gene is mediated through 2 distinct transactivation domains (ie, AF-1 and AF-2) of the receptor. The estrogen receptor also mediates gene transcription using different response elements (ie, AP-1) and other signal pathways. Recent advances in the molecular pharmacology of estrogen and estrogen receptors have resulted in the development of selective estrogen receptor modulators (eg, clomiphene, raloxifene, tamoxifen, toremifene), agents that bind and activate the estrogen receptor but that exhibit tissue-specific effects distinct from estrogen. Tissue-specific estrogen-agonist or -antagonist activity of these drugs appears to be related to structural differences in their estrogen receptor complex (eg, specifically the surface topography of AF-2 for raloxifene) compared with the estrogen (estradiol)-estrogen receptor complex. A second estrogen receptor also has been identified, and existence of at least 2 estrogen receptors (ER-alpha, ER-beta) may contribute to the tissue-specific activity of selective modulators. While the role of the estrogen receptor in bone, cardiovascular tissue, and the CNS continues to be studied, emerging evidence indicates that the mechanism of action of estrogen receptors in these tissues differs from the manner in which estrogen receptors function in reproductive tissue. /Estrogen General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3130
Intracellular cytosol-binding proteins for estrogens have been identified in estrogen-responsive tissues including the female genital organs, breasts, pituitary, and hypothalamus. The estrogen-binding protein complex (ie, cytosol-binding protein and estrogen) distributes into the cell nucleus where it stimulates DNA, RNA, and protein synthesis. The presence of these receptor proteins is responsible for the palliative response to estrogen therapy in women with metastatic carcinoma of the breast. /Estrogen General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3130
Estrogens have generally favorable effects on blood cholesterol and phospholipid concentrations. Estrogens reduce LDL-cholesterol and increase HDL-cholesterol concentrations in a dose-related manner. The decrease in LDL-cholesterol concentrations associated with estrogen therapy appears to result from increased LDL catabolism, while the increase in triglyceride concentrations is caused by increased production of large, triglyceride-rich, very-low-density lipoproteins (VLDLs); changes in serum HDL-cholesterol concentrations appear to result principally from an increase in the cholesterol and apolipoprotein A-1 content of HDL2- and a slight increase in HDL3-cholesterol. /Estrogen General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3130
For more Mechanism of Action (Complete) data for ESTRIOL (12 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
97
PharmaCompass offers a list of Estriol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Estriol manufacturer or Estriol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Estriol manufacturer or Estriol supplier.
PharmaCompass also assists you with knowing the Estriol API Price utilized in the formulation of products. Estriol API Price is not always fixed or binding as the Estriol Price is obtained through a variety of data sources. The Estriol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Estriol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Estriol, including repackagers and relabelers. The FDA regulates Estriol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Estriol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Estriol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Estriol supplier is an individual or a company that provides Estriol active pharmaceutical ingredient (API) or Estriol finished formulations upon request. The Estriol suppliers may include Estriol API manufacturers, exporters, distributors and traders.
click here to find a list of Estriol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Estriol DMF (Drug Master File) is a document detailing the whole manufacturing process of Estriol active pharmaceutical ingredient (API) in detail. Different forms of Estriol DMFs exist exist since differing nations have different regulations, such as Estriol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Estriol DMF submitted to regulatory agencies in the US is known as a USDMF. Estriol USDMF includes data on Estriol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Estriol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Estriol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Estriol Drug Master File in Japan (Estriol JDMF) empowers Estriol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Estriol JDMF during the approval evaluation for pharmaceutical products. At the time of Estriol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Estriol suppliers with JDMF on PharmaCompass.
A Estriol CEP of the European Pharmacopoeia monograph is often referred to as a Estriol Certificate of Suitability (COS). The purpose of a Estriol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Estriol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Estriol to their clients by showing that a Estriol CEP has been issued for it. The manufacturer submits a Estriol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Estriol CEP holder for the record. Additionally, the data presented in the Estriol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Estriol DMF.
A Estriol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Estriol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Estriol suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Estriol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Estriol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Estriol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Estriol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Estriol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Estriol suppliers with NDC on PharmaCompass.
Estriol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Estriol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Estriol GMP manufacturer or Estriol GMP API supplier for your needs.
A Estriol CoA (Certificate of Analysis) is a formal document that attests to Estriol's compliance with Estriol specifications and serves as a tool for batch-level quality control.
Estriol CoA mostly includes findings from lab analyses of a specific batch. For each Estriol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Estriol may be tested according to a variety of international standards, such as European Pharmacopoeia (Estriol EP), Estriol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Estriol USP).

