
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
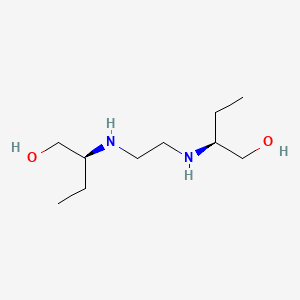

1. Dexambutol
2. Emb Fatol
3. Emb Hefa
4. Emb-fatol
5. Emb-hefa
6. Etambutol Llorente
7. Ethambutol Hydrochloride
8. Etibi
9. Hydrochloride, Ethambutol
10. Llorente, Etambutol
11. Miambutol
12. Myambutol
1. 74-55-5
2. Ethambutolum
3. Tibutol
4. Aethambutolum
5. D-ethambutol
6. (+)-s,s-ethambutol
7. (+)-ethambutol
8. (s,s)-ethambutol
9. Myambutol
10. (2s,2's)-2,2'-(ethane-1,2-diylbis(azanediyl))bis(butan-1-ol)
11. Etambutolo [dcit]
12. Ethambutol Hydrochloride
13. Etambutol [inn-spanish]
14. Ethambutolum [inn-latin]
15. S,s-ethambutol
16. Diambutol
17. Chebi:4877
18. (+)-2,2'-(ethylenediimino)di-1-butanol
19. Emb
20. Purderal
21. (+)-n,n'-bis(1-(hydroxymethyl)propyl)ethylenediamine
22. (2s)-2-[2-[[(2s)-1-hydroxybutan-2-yl]amino]ethylamino]butan-1-ol
23. Ethambutol (inn)
24. 8g167061qz
25. (2r)-2-[2-(1-hydroxybutan-2-ylamino)ethylamino]butan-1-ol
26. 1-butanol, 2,2'-(1,2-ethanediyldiimino)bis-, (2s,2's)-
27. 1-butanol,2,2'-(1,2-ethanediyldiimino)bis-, (2s,2's)-
28. Ethambutol [inn]
29. (2s,7s)-2,7-diethyl-3,6-diazaoctane-1,8-diol
30. (2s,2's)-2,2'-(ethane-1,2-diyldiimino)dibutan-1-ol
31. Etambutolo
32. D-2,2'-(ethylenediimino)di-1-butanol
33. D-2,2'-(ethylenediimino)bis(1-butanol)
34. Ethambutol, Racemic Mixture
35. 1-butanol, 2,2'-(ethylenediimino)di-, (+)-
36. D,n,n'-bis(1-hydroxymethylpropyl)ethylenediamine
37. D-n,n'-bis(1-hydroxymethylpropyl)ethylenediamine
38. Ethambutol [inn:ban]
39. (r)-2,2'-(1,2-ethanediyldiimino)bis-1-butanol
40. Ncgc00178864-03
41. 1-butanol, 2,2'-(1,2-ethanediyldiimino)bis-, (s-(r*,r*))-
42. Unii-8g167061qz
43. Hsdb 3078
44. Servambutol (tn)
45. 95e
46. Einecs 200-810-6
47. Spectrum_001058
48. Ethambutol [mi]
49. Spectrum2_001014
50. Spectrum3_000426
51. Spectrum4_000545
52. Spectrum5_000702
53. Ethambutol [hsdb]
54. Ethambutol [vandf]
55. 1-butanol, 2,2'-(1,2-ethanediyldiimino)bis-, (r)-
56. Myambutol (dihydrochloride)
57. Schembl3399
58. Ethambutol [who-dd]
59. Bspbio_002012
60. Kbiogr_001209
61. Kbioss_001538
62. Chembl44884
63. Divk1c_000561
64. Spbio_001167
65. Cl 40881 (dihydrochloride)
66. Dtxsid8023006
67. Kbio1_000561
68. Kbio2_001538
69. Kbio2_004106
70. Kbio2_006674
71. Kbio3_001232
72. Ninds_000561
73. Dtxsid901028179
74. Hy-b0535
75. Bdbm50448407
76. Zinc19364219
77. Db00330
78. Idi1_000561
79. Ncgc00178864-01
80. Ncgc00178864-04
81. Sbi-0051375.p003
82. C06984
83. D07925
84. D94801
85. E-3950
86. Ab00053473_04
87. Ab00053473_05
88. Q412318
89. (s,s)-2,2'-(1,2-ethanediyldiimino)bis-1-butanol
90. Brd-k93231391-300-03-1
91. (+)-(s,s)-2,2'-(1,2-ethylenediimino)-di-1-butanol
92. (2r*,2'r*)-2,2'-(ethane-1,2-diyldiimino)dibutan-1-ol
93. (2s,2s)-2,2-(ethane-1,2-diyldiimino)dibutan-1-ol
94. Ethambutol Dihydrochloride, Antibiotic For Culture Media Use Only
95. (2s)-2-[(2-{[(2s)-1-hydroxybutan-2-yl]amino}ethyl)amino]butan-1-ol
96. (2s)-2-[2-[[(1s)-1-(hydroxymethyl)propyl]amino]ethylamino]butan-1-ol
| Molecular Weight | 204.31 g/mol |
|---|---|
| Molecular Formula | C10H24N2O2 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 9 |
| Exact Mass | 204.183778013 g/mol |
| Monoisotopic Mass | 204.183778013 g/mol |
| Topological Polar Surface Area | 64.5 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 109 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Myambutol |
| PubMed Health | Ethambutol (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | MYAMBUTOL ethambutol hydrochloride is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium, including M. tuberculosis. The structural formula is:MYAMBUTOL 100 and 400 mg tab... |
| Active Ingredient | Ethambutol hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 400mg; 100mg |
| Market Status | Prescription |
| Company | Sti Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Myambutol |
| PubMed Health | Ethambutol (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | MYAMBUTOL ethambutol hydrochloride is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium, including M. tuberculosis. The structural formula is:MYAMBUTOL 100 and 400 mg tab... |
| Active Ingredient | Ethambutol hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 400mg; 100mg |
| Market Status | Prescription |
| Company | Sti Pharma |
Antitubercular Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Ethambutol is indicated in combination with other antituberculosis medications in the treatment of all forms of tuberculosis, including tuberculous meningitis, caused by Mycobacterium tuberculosis. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1334
Ethambutol is used in the treatment of atypical mycobacterial infections, such as Mycobacterium avium complex (MAC). /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1334
Appropriate studies on the relationship of age to the effects of ethambutol have not been performed in children up to 13 years of age. Ethambutol is generally not recommended in children whose visual acuity cannot be monitored (younger than 6 years of age). However, ethambutol should be considered for all children with organisms resistant to other medications, and in whom susceptibility to ethambutol has been demonstrated or is likely.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1335
The most important adverse effect of ethambutol is optic neuritis with decreases in visual acuity, constriction of visual fields, central and peripheral scotomas, and loss of red-green color discrimination. The extent of ocular toxicity appears to be related to the dose and duration of ethambutol therapy. However, such toxicity also has been reported rarely after only a few days of therapy with the drug, and may represent an idiosyncratic reaction.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551
Other adverse effects of ethambutol include dermatitis, pruritus, headache, malaise, dizziness, fever, mental confusion, disorientation, possible hallucinations, joint pain, and rarely anaphylactoid reactions. GI upset, abdominal pain, nausea, vomiting, and anorexia have also occurred occasionally with ethambutol. Peripheral neuritis, with numbness and tingling of the extremities, has been reported infrequently. Increased serum uric acid concentrations and precipitation of acute gout have occurred occasionally in patients receiving ethambutol and are probably the result of decreased renal clearance of urate. Transient impairment of liver function, as indicated by abnormal liver function test results, has also occurred. Cholestatic jaundice, which appeared to be caused by ethambutol, has been reported in at least one patient who received the drug both alone and in conjunction with streptomycin.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551
Visual testing should be performed prior to initiating ethambutol therapy and then periodically during therapy with the drug. Testing should be done monthly in patients receiving more than 15 mg/kg daily. Examinations should include ophthalmoscopy, finger perimetry, and testing of color discrimination. Patients developing adverse ocular effects during ethambutol therapy may show subjective visual symptoms either before or simultaneously with decreases in visual acuity. All patients receiving the drug should be questioned periodically about blurred vision and other subjective visual symptoms and should be instructed to report to their physicians any such changes as soon as they are noticed. If substantial changes in visual acuity occur, ethambutol should be discontinued immediately.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551
For more Drug Warnings (Complete) data for ETHAMBUTOL (10 total), please visit the HSDB record page.
Ethambutol is indicated in combination with other anti-tuberculosis drugs in the treatment of pulmonary tuberculosis. Ethambutol is commonly used in combination with [isoniazid], [rifampin], and [pyrazinamide].
FDA Label
Ethambutol is indicated in combination with other anti-tuberculosis drugs in the treatment of pulmonary tuberculosis. It has a long duration of action as it is administered daily, and a moderate therapeutic window. Patients should be counselled regarding the risk of optic neuritis and hepatic toxicity.
Antitubercular Agents
Drugs used in the treatment of tuberculosis. They are divided into two main classes: "first-line" agents, those with the greatest efficacy and acceptable degrees of toxicity used successfully in the great majority of cases; and "second-line" drugs used in drug-resistant cases or those in which some other patient-related condition has compromised the effectiveness of primary therapy. (See all compounds classified as Antitubercular Agents.)
J04AK02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J04 - Antimycobacterials
J04A - Drugs for treatment of tuberculosis
J04AK - Other drugs for treatment of tuberculosis
J04AK02 - Ethambutol
Absorption
Oral ethambutol is approximately 75-80% orally bioavailable. A 25 mg/kg oral dose of ethambutol reaches a Cmax of 2-5 g/mL, with a Tmax of 2-4 hours. In a separate study, the AUC0-8 varied from 6.3 5.5 h\*mg/L to 10.8 7.6 h\*mg/L depending on CYP1A2 genetic polymorphisms.
Route of Elimination
Ethambutol is 50% eliminated in the urine as the unmetabolized parent compound and 8-15% as inactive metabolites. 20-22% of a dose is eliminated unchanged in the feces.
Volume of Distribution
Patients coinfected with tuberculosis and HIV have an estimated ethambutol volume of distribution of 76.2 L.
Clearance
Patients coinfected with tuberculosis and HIV have an estimated ethambutol oral clearance of 77.4 L/h.
Approximately 75-80% of an oral dose of ethambutol hydrochloride is rapidly absorbed from the GI tract. Absorption is not substantially affected when the drug is administered with food. Following a single oral ethambutol hydrochloride dose of 25 mg/kg, peak serum ethambutol concentrations of 2-5 mcg/mL are attained within 2-4 hours; serum concentrations of the drug are undetectable 24 hours after the dose.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551
There is no evidence that accumulation of the drug occurs when ethambutol doses of 25 mg/kg are given once daily in patients with normal renal function. Serum concentrations of the drug are higher and accumulation may occur when ethambutol is used in patients with impaired renal function.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551
Ethambutol is widely distributed into most body tissues and fluids. Highest concentrations of the drug are found in erythrocytes, kidneys, lungs, and saliva; lower drug concentrations are found in ascitic fluid, pleural fluid, brain, and CSF. Peak intracellular concentrations of ethambutol in erythrocytes are about twice peak plasma concentrations and maintain this ratio for at least 24 hours after a single oral dose. In patients with meningitis, administration of an oral ethambutol hydrochloride dose of 25 mg/kg has produced peak CSF concentrations of the drug ranging from 0.15-2.0 ug/mL.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551
/Ethambutol/ does not penetrate intact meninges, but 10 to 50% may penetrate the meninges of patients with tuberculous meningitis. Volume of distribution is 1.6 liters per kg
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1334
For more Absorption, Distribution and Excretion (Complete) data for ETHAMBUTOL (9 total), please visit the HSDB record page.
Ethambutol is mainly oxidized by an aldehyde dehydrogenase to an aldehyde metabolite, followed by conversion to the dicarboxylic acid 2,2'-(ethylinediimino)di-butyric acid.
... Up to 15% is excreted in the form of two metabolites, an aldehyde and a dicarboxylic acid derivative.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1161
Ethambutol is partially inactivated in the liver by oxidation to an aldehyde intermediate, 2,2?-(ethylenediimino)-di-butyraldehyde, which is converted to the decarboxylic acid derivative, 2,2?-(ethylenediimino)-di-butyric acid.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551
Ethambutol has a half life of 3.3 hours in patients with normal renal function. In patients with renal failure, the half life could be 7 hours or longer.
The plasma half-life of ethambutol is approximately 3.3 hours in patients with normal renal function. The half-life is prolonged in patients with impaired renal or hepatic function. In patients with renal failure, the half-life may be 7 hours or longer.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551
Six normal adult volunteers were administered 15 mg/kg of ethambutol (EMB) by a constant-rate 1-hr infusion. Plasma and urine samples were collected up to 24 and 72 hr, respectively. ... Subsequent postinfusion EMB levels exhibited multiphasic decay. In the 12-hr period following infusion, EMB levels showed biexponential decay. However, 24-hr plasma levels in all subjects were observed to be higher than those predicted using a two-compartment body model. The alpha phase in these subjects had a mean half-life of 8.6 min while the half-life of the beta phase ranged from 2.5 to 3.6 hr (mean 3.1). The half-life of the gamma phase estimated from plasma data points between 12 and 24 hr averaged 1.2 +/- 3.6 hr. A terminal gamma t1/2 of 15.4 +/- 1.7 hr was calculated from 12-72 hr urine data. ... Plasma EMB clearance ranged from 7.47 to 8.87 mL/min/kg (mean 8.57). ...
PMID:7431225 Lee C et al; J Pharmacokinet Biopharm 8 (4): 335-46 (1980)
Ethambutol diffuses into _Mycobacterium_ cells. Once inside the cell, ethambutol inhibits the arabinosyltransferases (embA, embB, and embC), preventing formation of the cell wall components arabinogalactan and lipoarabinomannan, and preventing cell division. Decreased concentrations of arabinogalactan in the cell wall reduces the number of binding sites for mycolic acid, leading to the accumulation of mycolic acid, trehalose monomycolate, and trehalose dimycolate. Lipoarabinomannan is a component of a cell surface molecule involved in the interaction with host cells. Reduced levels of lipoarabinomannan may interfere with mycobacterial interaction with host cells.
Ethambutol is bacteriostatic in action. Although the exact mechanism of action has not been fully elucidated, the drug appears to inhibit the synthesis of one or more metabolites in susceptible bacteria resulting in impairment of cellular metabolism, arrest of multiplication, and cell death. Ethambutol is active against susceptible bacteria only when they are undergoing cell division.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 551

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
59
PharmaCompass offers a list of Ethambutol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ethambutol manufacturer or Ethambutol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ethambutol manufacturer or Ethambutol supplier.
PharmaCompass also assists you with knowing the Ethambutol API Price utilized in the formulation of products. Ethambutol API Price is not always fixed or binding as the Ethambutol Price is obtained through a variety of data sources. The Ethambutol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ethambutol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ethambutol, including repackagers and relabelers. The FDA regulates Ethambutol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ethambutol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ethambutol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ethambutol supplier is an individual or a company that provides Ethambutol active pharmaceutical ingredient (API) or Ethambutol finished formulations upon request. The Ethambutol suppliers may include Ethambutol API manufacturers, exporters, distributors and traders.
click here to find a list of Ethambutol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Ethambutol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ethambutol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ethambutol GMP manufacturer or Ethambutol GMP API supplier for your needs.
A Ethambutol CoA (Certificate of Analysis) is a formal document that attests to Ethambutol's compliance with Ethambutol specifications and serves as a tool for batch-level quality control.
Ethambutol CoA mostly includes findings from lab analyses of a specific batch. For each Ethambutol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ethambutol may be tested according to a variety of international standards, such as European Pharmacopoeia (Ethambutol EP), Ethambutol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ethambutol USP).
