
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
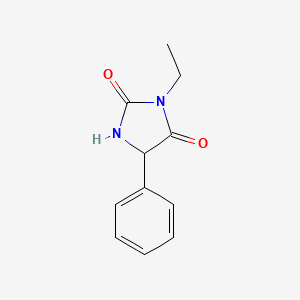

1. Peganone
1. Peganone
2. 86-35-1
3. 3-ethyl-5-phenylimidazolidine-2,4-dione
4. Accenon
5. 3-ethyl-5-phenylhydantoin
6. Ethotoine
7. Ethotoinum
8. Etotoina
9. Ethotoine [inn-french]
10. Ethotoinum [inn-latin]
11. Etotoina [inn-spanish]
12. 3-ethyl-5-phenylimidazolidin-2,4-dione
13. 1-ethyl-2,5-dioxo-4-phenylimidazolidine
14. 3-ethyl-5-phenyl-2,4-imidazolidinedione
15. 2,4-imidazolidinedione, 3-ethyl-5-phenyl-
16. Hydantoin, 3-ethyl-5-phenyl-
17. (+-)-3-ethyl-5-phenylhydantoin
18. Ac-695; Accenon
19. Nsc-760074
20. 3-ethyl-5-phenyl-imidazolidine-2,4-dione
21. Ethotoin (200 Mg)
22. 46qg38nc4u
23. Chebi:4888
24. (+/-)-3-ethyl-5-phenylhydantoin
25. Mfcd00072127
26. Pegoanone
27. Peganone (tn)
28. Accenon (tn)
29. Hsdb 3580
30. Einecs 201-665-1
31. Ethotoin (jan/usp/inn)
32. Brn 0188272
33. Unii-46qg38nc4u
34. Ethotoin [usp:inn:ban:jan]
35. Cas-86-35-1
36. Ncgc00016340-01
37. Ethotoin [hsdb]
38. Ethotoin [inn]
39. Ethotoin [jan]
40. Ethotoin [mi]
41. Ethotoin [vandf]
42. (.+/-.)-ethotoin
43. Prestwick0_000696
44. Prestwick1_000696
45. Prestwick2_000696
46. Prestwick3_000696
47. Dsstox_cid_3020
48. Ethotoin [mart.]
49. Ethotoin [usp-rs]
50. Ethotoin [who-dd]
51. Chembl1095
52. Dsstox_rid_76833
53. Dsstox_gsid_23020
54. Oprea1_374844
55. Schembl34301
56. Bspbio_000851
57. 5-24-08-00005 (beilstein Handbook Reference)
58. Mls002153961
59. Spbio_002772
60. Ethotoin [orange Book]
61. 2,4-imidazolidinedione, 3-ethyl-5-phenyl-, (+-)-
62. Bpbio1_000937
63. Gtpl7183
64. Dtxsid6023020
65. Ethotoin [usp Monograph]
66. Hms1570k13
67. Hms2097k13
68. Hms2232m06
69. Hms3371o03
70. Hms3714k13
71. Hms3887i05
72. Pharmakon1600-01505428
73. Hy-b1642
74. Tox21_110383
75. Nsc760074
76. Akos008947805
77. Ccg-213433
78. Db00754
79. Nsc 760074
80. Ncgc00179401-01
81. Ncgc00179401-03
82. Ns-02146
83. Smr001233300
84. 3-ethyl-5-phenyl-2,4-imidazolidinedione #
85. Ab00513897
86. Cs-0013593
87. C07839
88. D00708
89. Ab00513897_07
90. Sr-01000841832
91. Q4533122
92. Sr-01000841832-2
93. Z53845223
94. 1-ethyl-2-hydroxy-4-phenyl-4,5-dihydro-1h-imidazol-5-one
95. 2,4-imidazolidinedione, 3-ethyl-5-phenyl-, (+/-)-
| Molecular Weight | 204.22 g/mol |
|---|---|
| Molecular Formula | C11H12N2O2 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 204.089877630 g/mol |
| Monoisotopic Mass | 204.089877630 g/mol |
| Topological Polar Surface Area | 49.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 272 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Peganone |
| PubMed Health | Ethotoin (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | PEGANONE (ethotoin tablets, USP) is an oral antiepileptic of the hydantoin series and is chemically identified as 3-ethyl-5-phenyl-2,4-imidazolidinedione. It is represented by the following structural formula:PEGANONE tablets are available in a dosag... |
| Active Ingredient | Ethotoin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Recordati Rare |
| 2 of 2 | |
|---|---|
| Drug Name | Peganone |
| PubMed Health | Ethotoin (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | PEGANONE (ethotoin tablets, USP) is an oral antiepileptic of the hydantoin series and is chemically identified as 3-ethyl-5-phenyl-2,4-imidazolidinedione. It is represented by the following structural formula:PEGANONE tablets are available in a dosag... |
| Active Ingredient | Ethotoin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Recordati Rare |
Anticonvulsants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Hydantoin anticonvulsants are indicated in the suppression and control of tonic-clonic (grand mal) and simple or complex partial (psychomotor or temporal lobe) seizures. Ethotoin may be administered as a second-line agent when seizures have not been adequately controlled by the primary anticonvulsants and before proceeding to more toxic anticonvulsants. /Hydantoin anticonvulsants; Included in US product labeling./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 259
Hydantoin anticonvulsants are not indicated in the treatment of absence (petit mal) seizures, or as first-line treatment of febrile, hypoglycemic, or other metabolic seizures. When tonic-clonic (grand mal) seizures coexist with absence seizures, combined therapy may be necessary. /Hydantoin anticonvulsants; NOT included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 259
Ethotoin may be substituted for phenytoin without loss of seizure control for improvement of gum hyperplasia, or other side effects, during anticonvulsant therapy. Ethotoin doses are usually 4 to 6 times greater than those of phenytoin. /NOT included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 267
Ethotoin is contraindicated in patients with hepatic abnormalities or hematologic disorders.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2212
Although the etiologic role of ethotoin has not been definitely established, blood dyscrasias have been reported in patients receiving the drug, and clinicians should be alert to the possibility of their occurrence. Patients should be advised to report immediately any sign or symptom indicative of hematologic toxicity (e.g., sore throat, fever, malaise, petechiae, easy bruising, epistaxis). Complete blood cell counts should be performed before and at monthly intervals for several months after initiation of ethotoin therapy. The drug should be discontinued if marked depression of blood cell count occurs.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2212
Liver function tests should be performed in patients receiving ethotoin if there is clinical evidence of possible hepatic dysfunction. If signs of hepatotoxicity occur during ethotoin therapy, the drug should be discontinued.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2212
Ataxia and gingival hyperplasia have been reported only rarely during ethotoin therapy and usually only in patients receiving an additional hydantoin derivative. When ethotoin has replaced other hydantoin-derivative anticonvulsants, both of these reactions have subsided in some patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2212
For more Drug Warnings (Complete) data for ETHOTOIN (16 total), please visit the HSDB record page.
For the control of tonic-clonic (grand mal) and complex partial (psychomotor) seizures.
Ethotoin is a hydantoin derivative and anticonvulsant. Ethotoin exerts an antiepileptic effect without causing general central nervous system depression. The mechanism of action is probably very similar to that of phenytoin. The latter drug appears to stabilize rather than to raise the normal seizure threshold, and to prevent the spread of seizure activity rather than to abolish the primary focus of seizure discharges.
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AB - Hydantoin derivatives
N03AB01 - Ethotoin
Absorption
Fairly rapidly absorbed, however, the extent of oral absorption is not known.
Ethotoin is fairly rapidly absorbed from the GI tract following oral administration; the extent of absorption is not known.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2212
Therapeutic serum concentrations range from 15 to 50 ug/mL (74 to 245 umol/L) for ethotoin.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 260
Ethotoin and phenytoin are distributed into breast milk...
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 261
Limited data suggest that ethotoin and, to a lesser degree, 5-phenylhydantoin (the N-deethylated metabolite) may exhibit nonlinear pharmacokinetics following oral administration of single 500-, 1000-, and 1500-mg doses of ethotoin. The degree of nonlinearity may increase following oral administration of multiple doses (with a dosing interval of 4-6 hours) of ethotoin when compared with single doses, probably secondary to accumulation of the drug in plasma.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2213
For more Absorption, Distribution and Excretion (Complete) data for ETHOTOIN (9 total), please visit the HSDB record page.
Hepatic. The drug exhibits saturable metabolism with respect to the formation of N-deethyl and p-hydroxyl-ethotoin, the major metabolites.
Ethotoin is metabolized by the liver to p-hydroxylated and m-hydoxylated derivatives following N-deethylation; these metabolites are conjugated with glucuronic acid. The N-deethylated metabolite may also be metabolized to 2-phenylhydantoic acid. Ethotoin appears to exhibit saturable metabolism with respect to the formation of the p-hydroxylated and N-deethylated metabolites.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2212
The rate of hepatic biotransformation is increased in younger children, in pregnant women, in women during menses, and in patients with acute trauma; rate decreases with advancing age. /Hydantoin anticonvulsants/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 260
The urinary excretion pattern of ethotoin and five metabolites were examined in three patients receiving continuous treatment with ethotoin at two dose levels, in order to investigate the mechanism behind the dose-dependent kinetics of this anticonvulsant drug. The results suggest a partial saturation in the dealkylation process at high dose levels in three patients. A rough approximation of the Michaelis-Menten constants for different enzymatic processes was attempted. On the basis of the results obtained, the p-hydroxylation may be a saturable process. The dose-dependent kinetics of ethotoin in man seem to be explicable by the existence of partly saturable enzymatic pathways.
PMID:10117 Naestoft J et al; Clin Exp Pharmacol Physiol 3 (5): 453-9 (1976)
3 to 9 hours
At plasma concentrations less than about 8 ug/mL, ethotoin reportedly has an elimination half-life of 3-9 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2213
Ethotoin administration was 25 mg per kilogram in 5 patients. Ethotoin Tmax was 2 hours, with a T 1/2 of 5 hours. Saliva accurately represented the unbound fraction for ... /ethotoin/. Mean salivary levels (as percentage of total levels) were ... 54% for ethotoin.
PMID:42344 Troupin A et al; Ann Neurol 6 (5): 410-4 (1979)
The mechanism of action is probably very similar to that of phenytoin. The latter drug appears to stabilize rather than to raise the normal seizure threshold, and to prevent the spread of seizure activity rather than to abolish the primary focus of seizure discharges. Ethotoin inhibits nerve impulses in the motor cortex by lowering sodium ion influx, limiting tetanic stimulation.
The mechanism of action is not completely known, but it is thought to involve stabilization of neuronal membranes at the cell body, axon, and synapse and limitation of the spread of neuronal or seizure activity. ... Hydantoin anticonvulsants have an excitatory effect on the cerebellum, activating inhibitory pathways that extend to the cerebral cortex. This effect may also produce a reduction in seizure activity that is associated with an increased cerebellar Purkinje cell discharge. /Hydantoin anticonvulsants/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 259
Related Excipient Companies

Excipients by Applications
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
29
PharmaCompass offers a list of Ethotoin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ethotoin manufacturer or Ethotoin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ethotoin manufacturer or Ethotoin supplier.
PharmaCompass also assists you with knowing the Ethotoin API Price utilized in the formulation of products. Ethotoin API Price is not always fixed or binding as the Ethotoin Price is obtained through a variety of data sources. The Ethotoin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ethotoin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ethotoin, including repackagers and relabelers. The FDA regulates Ethotoin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ethotoin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Ethotoin supplier is an individual or a company that provides Ethotoin active pharmaceutical ingredient (API) or Ethotoin finished formulations upon request. The Ethotoin suppliers may include Ethotoin API manufacturers, exporters, distributors and traders.
click here to find a list of Ethotoin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ethotoin DMF (Drug Master File) is a document detailing the whole manufacturing process of Ethotoin active pharmaceutical ingredient (API) in detail. Different forms of Ethotoin DMFs exist exist since differing nations have different regulations, such as Ethotoin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ethotoin DMF submitted to regulatory agencies in the US is known as a USDMF. Ethotoin USDMF includes data on Ethotoin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ethotoin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ethotoin suppliers with USDMF on PharmaCompass.
Ethotoin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ethotoin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ethotoin GMP manufacturer or Ethotoin GMP API supplier for your needs.
A Ethotoin CoA (Certificate of Analysis) is a formal document that attests to Ethotoin's compliance with Ethotoin specifications and serves as a tool for batch-level quality control.
Ethotoin CoA mostly includes findings from lab analyses of a specific batch. For each Ethotoin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ethotoin may be tested according to a variety of international standards, such as European Pharmacopoeia (Ethotoin EP), Ethotoin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ethotoin USP).
