
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
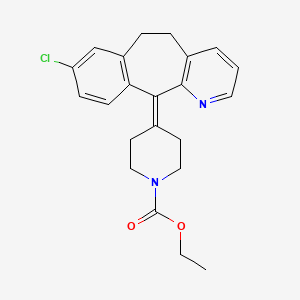

1. 4-(8-chloro-5,6-dihydro-11h-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylic Acid Ethyl Ester
2. Alavert
3. Claritin
4. Clarium
5. Sch 29851
6. Sch-29851
7. Sch29851
1. 79794-75-5
2. Claritin
3. Alavert
4. Loratidine
5. Clarityn
6. Lisino
7. Clarityne
8. Loracert
9. Loradex
10. Bonalerg
11. Claritine
12. Fristamin
13. Histaloran
14. Lertamine
15. Lorastine
16. Sch 29851
17. Civeran
18. Loranox
19. Versal
20. Anhissen
21. Sch-29851
22. Ethyl 4-(8-chloro-5,6-dihydro-11h-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxylate
23. Mfcd00672869
24. Loratyne
25. Ethyl 4-(8-chloro-5,6-dihydro-11h-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylate
26. Allertidin
27. Claratyne
28. Polaratyne
29. Restamine
30. Aerotina
31. Alerpriv
32. Biloina
33. Lesidas
34. Loradif
35. Lorantis
36. Loraver
37. Lorfast
38. Loritine
39. Lowadina
40. Nularef
41. Optimin
42. Sanelor
43. Sensibit
44. Sohotin
45. Velodan
46. 7ajo3bo7qn
47. Lergy
48. Pylor
49. Tadine
50. Chembl998
51. Zeos
52. Nsc-758628
53. Sinhistan Dy
54. Bedix Loratadina
55. Talorat Dy
56. Claritin Reditabs
57. Clarinase Reperabs
58. Mls000069647
59. 1-piperidinecarboxylic Acid, 4-(8-chloro-5,6-dihydro-11h-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-, Ethyl Ester
60. Ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene}piperidine-1-carboxylate
61. Loratadinum [latin]
62. Claritin Reditab
63. Loratadina [spanish]
64. Clarityne-d
65. Bay76-2211
66. 1-piperidinecarboxylic Acid, 4-(8-chloro-5,6-dihydro-11h-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-, Ethyl Ester
67. 1398065-63-8
68. Ethyl 4-(13-chloro-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene)piperidine-1-carboxylate
69. Ethyl 4-(8-chloro-5,6-dihydrobenzo[1,2]cyclohepta[2,4-b]pyridin-11-ylidene)piperidine-1-carboxylate
70. Children's Claritin
71. Ncgc00015619-09
72. Loratadinum
73. Loratadina
74. Smr000058255
75. Clarityne Dy Repetabs
76. Dsstox_cid_3224
77. Dsstox_rid_76931
78. Dsstox_gsid_23224
79. Loratadine Redidose
80. Ethyl 4-(8-chloro-5,6-dihydro-11h-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidinecarboxylate
81. Claritin Hives Relief
82. Bactimicina Allergy
83. Claritin (tn)
84. Claritin Hives Relief Reditab
85. Cas-79794-75-5
86. Hsdb 3578
87. Sr-01000075968
88. Unii-7ajo3bo7qn
89. Cronopen
90. Flonidan
91. Klaritin
92. Loratadine-form1
93. Topcare Childrens Allergy Relief
94. Loratadinei-p25
95. Loratadine,(s)
96. Loratadine [usan:usp:inn:ban]
97. Loratadine- Bio-x
98. Loratadine-form2-he
99. Spectrum_001496
100. Tocris-1944
101. Loratadine [mi]
102. Loratadine [inn]
103. Loratadine [jan]
104. Loratadinei-0deg(l13)
105. Opera_id_1868
106. Spectrum2_001584
107. Spectrum3_000740
108. Spectrum4_000177
109. Spectrum5_001650
110. Lopac-l-9664
111. Loratadine [hsdb]
112. Loratadine [usan]
113. Loratadine-d5(ethyl-d5)
114. Loratadinei-p80(l13)
115. Loratadine [vandf]
116. L 9664
117. Loratadine [mart.]
118. Loratadinei-m100(l13)
119. Loratadinei-m173(l13)
120. Schembl4596
121. Loratadine [usp-rs]
122. Loratadine [who-dd]
123. Lopac0_000680
124. Oprea1_027965
125. Regid_for_cid_3957
126. Bspbio_002300
127. Kbiogr_000693
128. Kbioss_001976
129. Loratadine (jan/usp/inn)
130. Mls000758260
131. Mls001148466
132. Mls001423984
133. Bidd:gt0198
134. Divk1c_000792
135. Spectrum1503712
136. Spbio_001548
137. Chebi:6538
138. Gtpl7216
139. Dtxsid2023224
140. Loratadine [ep Impurity]
141. Loratadine [orange Book]
142. Bdbm22876
143. Hms502h14
144. Kbio1_000792
145. Kbio2_001976
146. Kbio2_004544
147. Kbio2_007112
148. Kbio3_001520
149. Loratadine [ep Monograph]
150. Ninds_000792
151. Hms2051g11
152. Hms2090o18
153. Hms2093i15
154. Hms2235g23
155. Hms3262g21
156. Hms3268m16
157. Hms3371d13
158. Hms3393g11
159. Hms3412n06
160. Hms3654l17
161. Hms3676n06
162. Hms3714e09
163. Loratadine [usp Monograph]
164. Pharmakon1600-01503712
165. Zinc537931
166. Loratadine 0.1 Mg/ml In Methanol
167. Act04775
168. Amy15355
169. Bcp22338
170. Tox21_110185
171. Tox21_301532
172. Tox21_500680
173. Bbl010757
174. Ccg-39362
175. Dl-436
176. Nsc721075
177. Nsc758628
178. S1358
179. Stk574925
180. Akos005499513
181. Claritin-d Component Loratadine
182. Loratadine (desloratadine Impurity E)
183. Loratadine, >=98% (hplc), Powder
184. Tox21_110185_1
185. Ab06849
186. Ac-2086
187. Bcp9000858
188. Ccg-100786
189. Cs-0887
190. Db00455
191. Ks-1079
192. Lp00680
193. Nc00036
194. Nsc 721075
195. Nsc 758628
196. Nsc-721075
197. Sdccgsbi-0050659.p004
198. Idi1_000792
199. Ncgc00015619-01
200. Ncgc00015619-02
201. Ncgc00015619-03
202. Ncgc00015619-04
203. Ncgc00015619-05
204. Ncgc00015619-06
205. Ncgc00015619-07
206. Ncgc00015619-08
207. Ncgc00015619-10
208. Ncgc00015619-11
209. Ncgc00015619-12
210. Ncgc00015619-13
211. Ncgc00015619-26
212. Ncgc00023125-02
213. Ncgc00023125-04
214. Ncgc00023125-05
215. Ncgc00023125-06
216. Ncgc00023125-07
217. Ncgc00255171-01
218. Ncgc00261365-01
219. 4-(8-chloro-5,6-dihydro-11h-benzo[5,6]cycloheptal[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic Acid Ethyl Ester
220. Bl164637
221. Hy-17043
222. Loratadine Component Of Claritin-d
223. Nci60_041473
224. Sy052751
225. Bcp0726000007
226. Sbi-0050659.p003
227. Eu-0100680
228. Ft-0627976
229. L0223
230. Sw197416-3
231. D00364
232. Ab00053224-15
233. Ab00053224-16
234. Ab00053224_17
235. Ab00053224_18
236. 794l755
237. L000667
238. Q424049
239. Q-100833
240. Sr-01000075968-1
241. Sr-01000075968-3
242. Sr-01000075968-4
243. Sr-01000075968-6
244. Brd-k82795137-001-02-3
245. Brd-k82795137-001-10-6
246. Z1741979837
247. Loratadine, British Pharmacopoeia (bp) Reference Standard
248. Loratadine, European Pharmacopoeia (ep) Reference Standard
249. Loratadine, United States Pharmacopeia (usp) Reference Standard
250. Loratadine, Pharmaceutical Secondary Standard; Certified Reference Material
251. Loratadine For System Suitability, European Pharmacopoeia (ep) Reference Standard
252. 1-piperidenecarboxylic Acid,6-duhydro-11h-benzo [5,6]cyclohepta[1,2-b]-pyridin-11-ylidene)-, Ethyl Ester
253. 1-piperidinecarboxylic Acid,4-(8-chloro-5,6-dihydro-11h-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-, Ethyl Ester
254. 11-[n-(ethoxycarbonyl)-4-piperidylidene]-8-chloro-6,11-dihydro-5h-benzo-[5,6]cyclohepta[1,2-b]pyridine
255. 4-(8-chloro-5,6-dihydro-11h-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidinecarboxylic Acid Ethyl Ester
256. 4-(8-chloro-5,6-dihydro-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-piperidine-1-carboxylic Acid Ethyl Ester
257. 4-(8-chloro-5,6-dihydrobenzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxylic Acid Ethyl Ester
258. 8-chloro-11-(1-ethoxycarbonyl-4-piperidylidene)-6,11-dihydro-5h-benzo[5,6]cyclohepta[1,2-b]pyridine
259. 8-chloro-6,11-dihydro-11-(1- Ethoxycarbonyl-4-piperidylidene)-5h-benzo[5,6]cyclohepta[1,2-b]pyridine
260. 8-chloro-6,11-dihydro-11-(1-ethoxycarbonyl-4-piperidylidene)-5h-benzo[5,6]cyclohepta[1,2-b]pyridine
261. Ethyl 4-(8-chloro-5,6-dihydro-11h-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidine)-1-piperidinecarboxylate
262. Loratadine Impurity C;4-(4,8-dichloro-5,6-dihydro-11h-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylic Acid Ethyl Ester
| Molecular Weight | 382.9 g/mol |
|---|---|
| Molecular Formula | C22H23ClN2O2 |
| XLogP3 | 5.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 382.1448057 g/mol |
| Monoisotopic Mass | 382.1448057 g/mol |
| Topological Polar Surface Area | 42.4 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 569 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 18 | |
|---|---|
| Drug Name | Alavert |
| PubMed Health | Loratadine (By mouth) |
| Drug Classes | Respiratory Agent |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 10mg |
| Market Status | Over the Counter |
| Company | Pfizer |
| 2 of 18 | |
|---|---|
| Drug Name | Children's claritin |
| PubMed Health | Loratadine (By mouth) |
| Drug Classes | Respiratory Agent |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, chewable |
| Route | Oral |
| Strength | 5mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 3 of 18 | |
|---|---|
| Drug Name | Claritin |
| PubMed Health | Loratadine/Pseudoephedrine (By mouth) |
| Drug Classes | Antihistamine, Less-Sedating/Decongestant Combination, Antihistamine/Decongestant Combination |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet; Syrup; Capsule |
| Route | Oral |
| Strength | 1mg/ml; 10mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 4 of 18 | |
|---|---|
| Drug Name | Claritin hives relief |
| PubMed Health | Loratadine/Pseudoephedrine (By mouth) |
| Drug Classes | Antihistamine, Less-Sedating/Decongestant Combination, Antihistamine/Decongestant Combination |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 5 of 18 | |
|---|---|
| Drug Name | Claritin hives relief reditab |
| PubMed Health | Loratadine (By mouth) |
| Drug Classes | Respiratory Agent |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 10mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 6 of 18 | |
|---|---|
| Drug Name | Claritin reditabs |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 7 of 18 | |
|---|---|
| Drug Name | Claritin-d |
| Active Ingredient | Loratadine; pseudoephedrine sulfate |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 5mg; 120mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 8 of 18 | |
|---|---|
| Drug Name | Loratadine |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet; Syrup; Suspension; Tablet, orally disintegrating |
| Route | oral; Oral |
| Strength | 1mg/ml; 10mg; 5mg/5ml |
| Market Status | Over the Counter |
| Company | Ranbaxy; Wockhardt; Silarx; Actavis Labs Fl; Teva; Apotex; Taro; Sandoz; Pfizer; Perrigo; Mylan; Impax Labs |
| 9 of 18 | |
|---|---|
| Drug Name | Loratadine redidose |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 10mg |
| Market Status | Over the Counter |
| Company | Ranbaxy |
| 10 of 18 | |
|---|---|
| Drug Name | Alavert |
| PubMed Health | Loratadine (By mouth) |
| Drug Classes | Respiratory Agent |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 10mg |
| Market Status | Over the Counter |
| Company | Pfizer |
| 11 of 18 | |
|---|---|
| Drug Name | Children's claritin |
| PubMed Health | Loratadine (By mouth) |
| Drug Classes | Respiratory Agent |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, chewable |
| Route | Oral |
| Strength | 5mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 12 of 18 | |
|---|---|
| Drug Name | Claritin |
| PubMed Health | Loratadine/Pseudoephedrine (By mouth) |
| Drug Classes | Antihistamine, Less-Sedating/Decongestant Combination, Antihistamine/Decongestant Combination |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet; Syrup; Capsule |
| Route | Oral |
| Strength | 1mg/ml; 10mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 13 of 18 | |
|---|---|
| Drug Name | Claritin hives relief |
| PubMed Health | Loratadine/Pseudoephedrine (By mouth) |
| Drug Classes | Antihistamine, Less-Sedating/Decongestant Combination, Antihistamine/Decongestant Combination |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 14 of 18 | |
|---|---|
| Drug Name | Claritin hives relief reditab |
| PubMed Health | Loratadine (By mouth) |
| Drug Classes | Respiratory Agent |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 10mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 15 of 18 | |
|---|---|
| Drug Name | Claritin reditabs |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 16 of 18 | |
|---|---|
| Drug Name | Claritin-d |
| Active Ingredient | Loratadine; pseudoephedrine sulfate |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 5mg; 120mg |
| Market Status | Over the Counter |
| Company | Schering Plough |
| 17 of 18 | |
|---|---|
| Drug Name | Loratadine |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet; Syrup; Suspension; Tablet, orally disintegrating |
| Route | oral; Oral |
| Strength | 1mg/ml; 10mg; 5mg/5ml |
| Market Status | Over the Counter |
| Company | Ranbaxy; Wockhardt; Silarx; Actavis Labs Fl; Teva; Apotex; Taro; Sandoz; Pfizer; Perrigo; Mylan; Impax Labs |
| 18 of 18 | |
|---|---|
| Drug Name | Loratadine redidose |
| Active Ingredient | Loratadine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 10mg |
| Market Status | Over the Counter |
| Company | Ranbaxy |
Anti-Allergic Agents; Antipruritics; Histamine H1 Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Antihistamines are indicated in the prophylactic and symptomatic treatment of perennial and seasonal allergic rhinitis, vasomotor rhinitis, and allergic conjunctivitis due to inhalant allergens and foods. /Antihistamines; Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 324
Antihistamines are indicated for the symptomatic treatment of pruritus associated with allergic reactions and of mild, uncomplicated allergic skin manifestations of urticaria and angioedema, in dermatographism, and in urticaria associated with transfusions. /Antihistamines; Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 324
Antihistamines are also use in the treatment of pruritus associated with pityriasis rosea. /Antihistamines; NOT included in US or Canadian product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 324
For more Therapeutic Uses (Complete) data for LORATADINE (19 total), please visit the HSDB record page.
Small amounts of antihistamines are distributed into breast milk; use is not recommended in nursing mothers because of the risk of adverse effects, such as unusual excitement or irritability, in infants. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 327
Use is not recommended in newborn or premature infants because this age group has an increased susceptibility to anticholinergic side effects, such as central nervous system (CNS) excitation, and an increased tendency toward convulsions. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 327
A paradoxical reaction characterized by hyperexcitability may occur in children taking antihistamines. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 327
Dizziness, sedation, confusion, and hypotension may be more likely to occur in geriatric patients taking antihistamines. A paradoxical reaction characterized by hyperexcitability may occur in geriatric patients taking antihistamines. Geriatric patients are especially susceptible to the anticholinergic side effects, such as dryness of mouth and urinary retention (especially in males), of the antihistamines. If these side effects occur and continue or are severe, medication should probably be discontinued. /Antihistamines/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 327
For more Drug Warnings (Complete) data for LORATADINE (9 total), please visit the HSDB record page.
Loratadine is a 2nd generation antihistamine and is used to manage symptoms of allergic rhinitis, wheal formation, urticaria, and other allergic dermatologic conditions.
Like other 2nd generation antihistamines, loratadine is selective for peripheral H1 receptors. Loratadine does not penetrate effectively into the central nervous system and has poor affinity for CNS H1-receptors. These qualities result in a lack of CNS depressant effects such as drowsiness, sedation, and impaired psychomotor function.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
Histamine H1 Antagonists, Non-Sedating
A class of non-sedating drugs that bind to but do not activate histamine receptors (DRUG INVERSE AGONISM), thereby blocking the actions of histamine or histamine agonists. These antihistamines represent a heterogenous group of compounds with differing chemical structures, adverse effects, distribution, and metabolism. Compared to the early (first generation) antihistamines, these non-sedating antihistamines have greater receptor specificity, lower penetration of BLOOD-BRAIN BARRIER, and are less likely to cause drowsiness or psychomotor impairment. (See all compounds classified as Histamine H1 Antagonists, Non-Sedating.)
R06AX13
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AX - Other antihistamines for systemic use
R06AX13 - Loratadine
Absorption
Loratadine is rapidly absorbed and achieves peak plasma concentration in 1-2 hours, while it's main metabolite achieves peak plasma concentration in 3-4 hours. In the rapid dissolve formulation, the pharmacokinetic parameters of loratadine are as follows: Cmax = 2.56 ng/ml, Tmax = 1.14 hrs, AUC = 6.14 ng x hr/ml. In the rapid dissolve formulation, the pharmacokinetic parameters of descarboethoxyloratadine are as follows: Cmax = 3.72 ng/ml, Tmax = 1.97 hr, AUC = 49.1 ng x hr/ml. In the conventional formulation, the pharmacokinetic parameters of loratadine are as follows: Cmax = 2.11 ng/ml, Tmax = 1.00 hr, AUC = 4.64 ng x hr/ml In the conventional formulation, the pharmacokinetic parameters of descarboethoxyloratadine are as follows: Cmax = 3.66 ng/ml, Tmax = 1.97 hr, AUC = 48.4 ng x hr/ml
Route of Elimination
Over a 10 day period, 40% of loratadine is excreted in the urine, and 42% is eliminated in the faeces.
Volume of Distribution
The volume of distribution of loratadine is 120 L/Kg.
Clearance
The clearance of loratadine after single oral doses of 20 mg and 40 mg are 12 L/h/kg and 9 L/h/kg respectively. P-glycoprotein is involved in the clearance of many 2nd generation antihistamines, including loratadine, from the central nervous system. 1st generation antihistamines are not cleared by P-glycoprotein, which may help explain why they have a different central nervous system adverse effect profile compared to their 2nd generation counterparts. It appears that an antihistamine with higher affinity for p-glycoprotein will have a lower incidence of CNS adverse effects.
H1 antagonists are eliminated more rapidly by children than by adults and more slowly in those with severe liver disease. /H1 Receptor Antagonists/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 590
The H1 antagonists are well absorbed from the gastrointestinal tract. Following oral administration, peak plasma concentrations are achieved in 2 to 3 hours ... . /H1 Receptor Antagonists/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 588
Approximately 80% of the total dose administered can be found equally distributed between urine and feces in the form of metabolic products after 10 days.
PDR; Physicians' Desk Reference. 50th Ed. Montvale, NJ: Medical Economics Co. p.2349 (1996)
Whole body autoradiographic studies in rats and monkeys, radiolabeled tissue distribution studies in mice and rats, and in vivo radioligand studies in mice have shown that neither loratadine nor its metabolites readily cross the blood-brain barrier. Radioligand binding studies with guinea pig pulmonary and brain H1-receptors indicate that there was preferential binding to peripheral versus central nervous system H1-receptors.
PDR; Physicians' Desk Reference. 50th Ed. Montvale, NJ: Medical Economics Co. p.2349 (1996)
Unlike other currently available antihistamines, second generation antihistamines such as ... loratadine appear to distribute poorly or not appreciably into the CNS at usual dosages.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 3
Loratadine undergoes extensive first pass metabolism in the liver and is primarily metabolized by CYP3A4, CYP2D6, CYP1A1 and CYP2C19. Less involved CYP enzymes include CYP1A2, CYP2B6, CYP2C8, CYP2C9 and CYP3A5. CYP3A4 and CYP2D6 are mainly responsible for metabolizing loratadine to descarboethoxyloratadine. This primary metabolite is 4 times more pharmacologically active than loratadine. In addition, a study demonstrates that descarboethoxyloratadine is first glucuronidated by UGT2B10, then hydroxylated by CYP2C8 to form 3-hydroxydesloratadine. Further glucuronidation of 3-hydroxydesloratadine facilitates excretion.
The second generation H1 antagonists astemizole, loratadine,and terfenadine are rapidly absorbed from the gastrointestinal tract and metabolized in the liver to active metabolites by the hepatic microsomal p450 system.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 590
Pharmacokinetic studies following single and multiple oral doses of loratadine in 115 volunteers showed that loratadine is rapidly absorbed and extensively metabolized to an active metabolite (descarboethoxyloratadine).
PDR; Physicians' Desk Reference. 50th Ed. Montvale, NJ: Medical Economics Co. p.2349 (1996)
In vitro studies with human liver microsomes indicate that loratadine is metabolized to descarboethoxyloratadine predominately by p450 CYP3A4 and, to a lesser extent, by p450 CYP2D6.
PDR; Physicians' Desk Reference. 50th Ed. Montvale, NJ: Medical Economics Co. p.2349 (1996)
H1 receptor antagonists are among the many drugs that induce hepatic microsomal enzymes, and they may facilitate their own metabolism. /H1 Receptor Antagonists/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 590
The non-sedating anti-histamine, loratadine ... was admin orally in the diet to mature male rats at dosages of 4, 10 and 25 mg/kg/day for 2 wk. The effects of these treatments on liver microsomal cytochrome P450 were evaluated by immunochemical and biochemical techniques, and were compared with the effects of treating rats with three different inducers of cytochrome P450, namely phenobarbital, 3-methylcholanthrene and dexamethasone. Treatment of rats with loratadine caused a dose dependent incr in the levels of P450 2Bl and 2B2, the major phenobarbital inducible P450 enzymes, as determined by Western immunoblotting. At the highest dosage tested, loratadine was less effective than phenobarbital as an inducer of 2Bl and 2B2, although the induction of these proteins could be detected immunochemically even at the lowest dosage of loratadine tested. Consistent with these observations, treatment of rats with loratadine caused a dose dependent incr in the rate of two reactions that are catalyzed predominantly by 2Bl/2, namely testosterone 16 beta-hydroxylation and 7-pentoxyresorufin O-dealkylation. At the highest dosage tested, loratadine caused a 7.3- and 8.5-fold incr in the rate of testosterone 16 beta-hydroxylation and 7-pentoxyresorufin O-dealkylation, respectively, compared with a and 45-fold incr caused by phenobarbital treatment. Treatment of rats with loratadine caused a 1.4 to 2.0-fold incr in the 2 beta-, 6 beta- and 15 beta-hydroxylation of testosterone, which was associated with a similar incr in the levels of immunoreactive P450 3Al and/or 3A2. As an inducer of P450 3Al/2, loratadine was slightly less effective than phenobarbital, and was considerably less effective than dexamethasone, which caused a 10- to 33-fold increase in testosterone 2 beta-, 6 beta- and 15 beta-hydroxylase activity. At the dosages tested, loratadine did not increase the levels of P450 lAl, the major 3-methylcholanthrene inducible P450 enzyme, as determined by Western immunoblotting. The rate of 7-ethoxyresorufin O-dealkylation, which is catalyzed predominantly by P450 lAl, incr 1.9-fold after loratidine treatment, but this incr was less than that caused by phenobarbital treatment (2.2-fold), and was considerably less than that caused by 3-methylcholanthrene treatment (33-fold). The effects of treating mature male mice with loratadine on liver microsomal cytochrome P450 resembled the effects observed in rats. These results indicate that loratadine is a phenobarbital type inducer of liver microsomal cytochrome P450 in rats and mice.
PMID:1534660 Parkinson A, et al; Biochem Pharmacol 43 (10): 2169-80 (1992)
Loratadine has known human metabolites that include Desloratadine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half life is approximately 10 hours for loratadine and 20 hours for descarboethoxyloratadine.
The mean elimination half-lives found in studies in normal adult subjects (n= 54) were 8.4 hours (range= 3 to 20 hours) for loratadine and 28 hours (range= 8.8 to 92 hours) for the major active metabolites (descarboethoxyloratadine).
PDR; Physicians' Desk Reference. 50th Ed. Montvale, NJ: Medical Economics Co. p.2349 (1996)
Histamine release is a key mediator in allergic rhinitis and urticaria. As a result, loratadine exerts it's effect by targeting H1 histamine receptors. Loratadine binds to H1 histamine receptors found on the surface of epithelial cells, endothelial cells, eosinophils, neutrophils, airway cells, and vascular smooth muscle cells among others. H1 histamine receptors fall under the wider umbrella of G-protein coupled receptors, and exist in a state of equilibrium between the active and inactive forms. Histamine binding to the H1-receptor facilitates cross linking between transmembrane domains III and V, stabilizing the active form of the receptor. On the other hand, antihistamines bind to a different site on the H1 receptor favouring the inactive form. Hence, loratadine can more accurately be classified as an "inverse agonist" as opposed to a "histamine antagonist", and can prevent or reduce the severity of histamine mediated symptoms.
All of the available H1 receptor antagonists are reversible, competitive inhibitors of the interaction of histamine with H1 receptors. /H1 Receptor Antagonists/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 587
H1 antagonists inhibit most responses of smooth muscle to histamine. /H1 Antagonists Receptors/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 587
Within the vascular tree, the H1 antagonists inhibit both the vasoconstrictor effects of histamine and, to a degree, the more rapid vasodilator effects that are mediated by H1 receptors on endothelial cells. /H1 Receptor Antagonists/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 588
H1 antagonists strongly block the action of histamine that results in increased capillary permeability and formation of edema and wheal. /H1 Receptor Antagonists/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 588
For more Mechanism of Action (Complete) data for LORATADINE (6 total), please visit the HSDB record page.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16025
Submission : 2002-05-28
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6626
Submission : 1986-10-15
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-09-28
Pay. Date : 2013-09-24
DMF Number : 12650
Submission : 1997-09-11
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13032
Submission : 1998-06-18
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9830
Submission : 1992-07-22
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11803
Submission : 1995-12-19
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15251
Submission : 2001-01-17
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-05-26
Pay. Date : 2014-07-02
DMF Number : 14155
Submission : 1999-05-26
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15018
Submission : 2000-08-28
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
 Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Certificate Number : CEP 2023-059 - Rev 00
Status : Valid
Issue Date : 2024-07-30
Type : Chemical
Substance Number : 2124
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2021-294 - Rev 00
Status : Valid
Issue Date : 2022-10-11
Type : Chemical
Substance Number : 2124

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2007-284 - Rev 03
Status : Valid
Issue Date : 2024-01-04
Type : Chemical
Substance Number : 2124

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2007-218 - Rev 00
Status : Valid
Issue Date : 2013-12-19
Type : Chemical
Substance Number : 2124

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Loratadine, Milled, Micronised
Certificate Number : CEP 2007-348 - Rev 05
Status : Valid
Issue Date : 2024-09-16
Type : Chemical
Substance Number : 2124

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2008-172 - Rev 02
Status : Valid
Issue Date : 2021-03-04
Type : Chemical
Substance Number : 2124

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2007-283 - Rev 01
Status : Valid
Issue Date : 2017-03-01
Type : Chemical
Substance Number : 2124

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2008-282 - Rev 02
Status : Valid
Issue Date : 2019-06-26
Type : Chemical
Substance Number : 2124

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2007-171 - Rev 05
Status : Valid
Issue Date : 2024-07-11
Type : Chemical
Substance Number : 2124

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2009-009 - Rev 03
Status : Valid
Issue Date : 2023-01-30
Type : Chemical
Substance Number : 2124

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
2-Cyano-3-[2(3-chloro phenyl) ethyl]pyridine (L-5)
CAS Number : 31255-57-9
End Use API : Loratadine
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
CAS Number : 5570-77-4
End Use API : Loratadine
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
8-Chloro-6,11-dihydro-11-(1-methyl-4-piperidinylid...
CAS Number : 38092-89-6
End Use API : Loratadine
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
CAS Number : 1445-73-4
End Use API : Loratadine
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
CAS Number : 29976-53-2
End Use API : Loratadine
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
CAS Number : 5570-77-4
End Use API : Loratadine
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
CAS Number : 1445-73-4
End Use API : Loratadine
About The Company : Vamsi Labs, established in 1991, is a leading Indian API manufacturer. Specializing in anti-asthmatic, anti-migraine & anti-psychotic APIs, it caters to domesti...
CAS Number : 5570-77-4
End Use API : Loratadine
About The Company : Vamsi Labs, established in 1991, is a leading Indian API manufacturer. Specializing in anti-asthmatic, anti-migraine & anti-psychotic APIs, it caters to domesti...
3-[2-(3-ChIorophenyI)ethyI]-2-pyridinecarbonitriIe
CAS Number : 31255-57-9
End Use API : Loratadine
About The Company : Aptra Synthesis Private Limited, is a reliable and vertically integrated manufacturer of APIs and intermediates, boasting state-of-the-art manufacturing facilit...
CAS Number : 38092-89-6
End Use API : Loratadine
About The Company : Aptra Synthesis Private Limited, is a reliable and vertically integrated manufacturer of APIs and intermediates, boasting state-of-the-art manufacturing facilit...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?