
Synopsis
Synopsis
0
VMF
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Ay 24,236
2. Ay 24236
3. Ay-24,236
4. Ay-24236
5. Etodolac, (+-)-isomer
6. Etodolac, (-)-isomer
7. Etodolac, (s)-isomer
8. Etodolac, Monosodium Salt
9. Etodolac, Monosodium Salt, (+-) Isomer
10. Etodolac, Monosodium Salt, (s)-isomer
11. Etodolic Acid
12. Lodine
13. Ramodar
14. Ultradol
1. 41340-25-4
2. Etodolic Acid
3. Lodine
4. Ultradol
5. Lodine Xl
6. Ay-24236
7. Edolan
8. Ramodar
9. Ay-24,236
10. Etodolaco
11. Etodolacum
12. Tedolan
13. Zedolac
14. Rac-etodolac
15. Ay 24236
16. Eccoxolac
17. Flancox
18. Napilac
19. 2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetic Acid
20. Lodin Xl
21. 1,8-diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indole-1-acetic Acid
22. Pyrano[3,4-b]indole-1-acetic Acid, 1,8-diethyl-1,3,4,9-tetrahydro-
23. (rs)-etodolic Acid
24. 1,3,4,9-tetrahydro-1,8-diethylpyrano(3,4-b)indole-1-acetic Acid
25. 1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic Acid
26. 2-(1,8-diethyl-4,9-dihydro-3h-pyrano[3,4-b]indol-1-yl)acetic Acid
27. Chembl622
28. Nsc-282126
29. (1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetic Acid
30. Pyrano(3,4-b)indole-1-acetic Acid, 1,8-diethyl-1,3,4,9-tetrahydro-
31. Mls000028474
32. Chebi:4909
33. Rak-591
34. 2-{1,8-diethyl-1h,3h,4h,9h-pyrano[3,4-b]indol-1-yl}acetic Acid
35. (1,8-diethyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indol-1-yl)-acetic Acid
36. Nih-9918
37. Nsc282126
38. Smr000058443
39. Ay-24-236
40. Dsstox_cid_615
41. (+-)-1,8-diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indole-1-acetic Acid
42. (rs)-2-(1,8-diethyl-4,9-dihydro-3h-pyrano[3,4-b]indol-1-yl)acetic Acid
43. Etodolacum [inn-latin]
44. Dsstox_rid_75691
45. Dsstox_gsid_20615
46. Etodolaco [inn-spanish]
47. 2m36281008
48. Hypen
49. 1,8-diethyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indole-1-acetic Acid
50. 1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-ylacetic Acid
51. (+/-)-1,8-diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indole-1-acetic Acid
52. Ccris 3923
53. Sr-01000000100
54. Nsc 282126
55. Etogesic
56. Osteluc
57. Etodolac O
58. Etodlic Acid
59. Lodine Sr
60. Etodolac [usan:usp:inn:ban]
61. Etodolac- Bio-x
62. Ncgc00016849-01
63. Etodolac,(s)
64. Prestwick_209
65. Cas-41340-25-4
66. Lodine (tn)
67. Mfcd00133313
68. Unii-2m36281008
69. Etodolac Assay Standard
70. Spectrum_001244
71. Etodolac [usan]
72. Etodolac [inn]
73. Etodolac [jan]
74. Etodolac [mi]
75. Etodolac [vandf]
76. Opera_id_1774
77. Prestwick0_000231
78. Prestwick1_000231
79. Prestwick2_000231
80. Prestwick3_000231
81. Spectrum2_001387
82. Spectrum3_001429
83. Spectrum4_000410
84. Spectrum5_001347
85. Etodolac [mart.]
86. Etodolac [usp-rs]
87. Etodolac [who-dd]
88. Schembl3903
89. Lopac0_000479
90. (+/-)-etodolac
91. Bspbio_000301
92. Bspbio_003138
93. Kbiogr_000680
94. Kbioss_001724
95. Mls001077315
96. Mls006011566
97. Divk1c_000147
98. Etodolac [green Book]
99. Spectrum1501005
100. Etodolac (jp17/usp/inn)
101. Spbio_001374
102. Spbio_002222
103. Etodolac [orange Book]
104. Bpbio1_000333
105. Gtpl7185
106. Etodolac [ep Monograph]
107. Etodolac For Peak Identification
108. Dtxsid9020615
109. Etodolac [usp Monograph]
110. Hms500h09
111. Kbio1_000147
112. Kbio2_001724
113. Kbio2_004292
114. Kbio2_006860
115. Kbio3_002358
116. Ninds_000147
117. Hms1568p03
118. Hms1921b09
119. Hms2092o20
120. Hms2095p03
121. Hms2231d04
122. Hms3259p10
123. Hms3261o20
124. Hms3374g09
125. Hms3712p03
126. Pharmakon1600-01501005
127. Bcp28411
128. Tox21_110644
129. Tox21_202218
130. Tox21_500479
131. Bdbm50016799
132. Ccg-39005
133. Nsc757821
134. Pyrano(3,4-b)indole-1-acetic Acid, 1,3,4,9-tetrahydro-1,8-diethyl-
135. Akos015838610
136. Pyrano(3,4-b)indole-1-acetic Acid, 1,8-diethyl-1,3,4,9-tetrahydro-(+-)-
137. Tox21_110644_1
138. Ac-4231
139. Cs-0832
140. Db00749
141. Lp00479
142. Nc00718
143. Nsc-757821
144. Sdccgsbi-0050463.p004
145. Etodolac 100 Microg/ml In Acetonitrile
146. Idi1_000147
147. Ncgc00015399-03
148. Ncgc00015399-04
149. Ncgc00015399-05
150. Ncgc00015399-06
151. Ncgc00015399-07
152. Ncgc00015399-08
153. Ncgc00015399-09
154. Ncgc00015399-11
155. Ncgc00015399-12
156. Ncgc00015399-13
157. Ncgc00015399-15
158. Ncgc00015399-16
159. Ncgc00015399-26
160. Ncgc00089769-02
161. Ncgc00089769-03
162. Ncgc00089769-04
163. Ncgc00089769-05
164. Ncgc00089769-06
165. Ncgc00089769-07
166. Ncgc00259767-01
167. Ncgc00261164-01
168. Br164431
169. Hy-76251
170. Sbi-0050463.p003
171. Ab00052194
172. E0858
173. Eu-0100479
174. Ft-0607046
175. Ft-0668424
176. Ft-0668425
177. C06991
178. D00315
179. E 0516
180. Pyrano[3, 1,8-diethyl-1,3,4,9-tetrahydro-
181. Ab00052194_17
182. 340e254
183. Q-201099
184. Q2465218
185. Sr-01000000100-2
186. Sr-01000000100-4
187. Sr-01000000100-7
188. Brd-a74667430-001-05-3
189. Brd-a74667430-001-15-2
190. 1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic Acid
191. Etodolac, European Pharmacopoeia (ep) Reference Standard
192. F2173-0681
193. Z1820178466
194. Etodolac, United States Pharmacopeia (usp) Reference Standard
195. 1,8-diethyl-1,3,4,9-tetrahydropyranol[3,4-b]indole-1-acetic Acid
196. 1,8-diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indol-1-acetic Acid
197. 2-(1,8-diethyl-4,9-dihydro-3h-pyrano[3,4-b]indol-1-yl)acetic Acid.
198. Pyrano(3,4,b)indole-1-acetic Acid, 1,3,4,9-tetrahydro-1,8-diethyl-
199. Ay 24,236; Ay 24236;ay-24,236; Ay-24236; Ay24,236; Ay24236
200. Etodolac For Peak Identification, European Pharmacopoeia (ep) Reference Standard
201. Pyrano(3,4-b)indole-1-acetic Acid, 1,8-diethyl-1,3,4,9-tetrahydro-(+/-)-
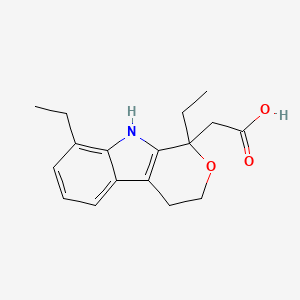
| Molecular Weight | 287.35 g/mol |
|---|---|
| Molecular Formula | C17H21NO3 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 287.15214353 g/mol |
| Monoisotopic Mass | 287.15214353 g/mol |
| Topological Polar Surface Area | 62.3 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 400 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Etodolac |
| PubMed Health | Etodolac (By mouth) |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Etodolac capsules and tablets, USP are members of the pyranocarboxylic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each tablet and capsule contains etodolac for oral administration. Etodolac is a racemic mixture of [+]S and [-]R-enan... |
| Active Ingredient | Etodolac |
| Dosage Form | Tablet, extended release; Tablet; Capsule |
| Route | Oral |
| Strength | 300mg; 200mg; 600mg; 500mg; 400mg |
| Market Status | Prescription |
| Company | Ani Pharms; Teva; Apotex; Taro; Taro Pharm Inds; Zydus Pharms Usa; Sandoz; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Etodolac |
| PubMed Health | Etodolac (By mouth) |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Etodolac capsules and tablets, USP are members of the pyranocarboxylic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each tablet and capsule contains etodolac for oral administration. Etodolac is a racemic mixture of [+]S and [-]R-enan... |
| Active Ingredient | Etodolac |
| Dosage Form | Tablet, extended release; Tablet; Capsule |
| Route | Oral |
| Strength | 300mg; 200mg; 600mg; 500mg; 400mg |
| Market Status | Prescription |
| Company | Ani Pharms; Teva; Apotex; Taro; Taro Pharm Inds; Zydus Pharms Usa; Sandoz; Mylan |
For acute and long-term management of signs and symptoms of osteoarthritis and rheumatoid arthritis, as well as for the management of pain.
FDA Label
Etodolac is an anti-inflammatory agent with analgesic and antipyretic properties. It is used to treat osteoarthritis, rheumatoid arthritis and control acute pain. The therapeutic effects of etodolac are achieved via inhibition of the synthesis of prostaglandins involved in fever, pain, swelling and inflammation. Etodolac is administered as a racemate. As with other NSAIDs, the S-form has been shown to be active while the R-form is inactive. Both enantiomers are stable and there is no evidence of R- to S- conversion _in vivo_.
Cyclooxygenase 2 Inhibitors
A subclass of cyclooxygenase inhibitors with specificity for CYCLOOXYGENASE-2. (See all compounds classified as Cyclooxygenase 2 Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AB - Acetic acid derivatives and related substances
M01AB08 - Etodolac
Absorption
Based on mass balance studies, the systemic bioavailability of etodolac from either the tablet or capsule formulation is at least 80%.
Route of Elimination
It is not known whether etodolac is excreted in human milk; however, based on its physical-chemical properties, excretion into breast milk is expected. Etodolac is extensively metabolized in the liver. The hydroxylated-etodolac metabolites undergo further glucuronidation followed by renal excretion and partial elimination in the feces (16% of dose). Approximately 1% of a etodolac dose is excreted unchanged in the urine with 72% of the dose excreted into urine as parent drug plus metabolite.
Volume of Distribution
390 mL/kg
Clearance
Oral cl=49.1 mL/h/kg [Normal healthy adults]
Oral cl=49.4 mL/h/kg [Healthy males (18-65 years)]
Oral cl=35.7 mL/h/kg [Healthy females (27-65 years)]
Oral cl=45.7 mL/h/kg [Eldery (>65 years)]
Oral cl=58.3 mL/h/kg [Renal impairement (46-73 years)]
Oral cl=42.0 mL/h/kg [Hepatic impairement (34-60 years)]
Etodolac is extensively metabolized in the liver. Renal elimination of etodolac and its metabolites is the primary route of excretion (72%). Metabolites found in urine (with percents of the administered dose) are: unchanged etodolac (1%), etodolac glucuronide (13%), hydroxylated metabolites (6-, 7-, and 8-OH; 5%), hydroxylated metabolite glucuronides (20%), and unidentified metabolites (33%). Fecal excretion accounts for 16% of its elimination.
Etodolac has known human metabolites that include (2S,3S,4S,5R)-6-[2-(1,8-Diethyl-4,9-dihydro-3H-pyrano[3,4-b]indol-1-yl)acetyl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Terminal t1/2, 7.3 ± 4.0 hours. Distribution t1/2, 0.71 ± 0.50 hours
Similar to other NSAIDs, the anti-inflammatory effects of etodolac result from inhibition of the enzyme cycooxygenase (COX). This decreases the synthesis of peripheral prostaglandins involved in mediating inflammation. Etodolac binds to the upper portion of the COX enzyme active site and prevents its substrate, arachidonic acid, from entering the active site. Etodolac was previously thought to be a non-selective COX inhibitor, but it is now known to be 5 50 times more selective for COX-2 than COX-1. Antipyresis may occur by central action on the hypothalamus, resulting in peripheral dilation, increased cutaneous blood flow, and subsequent heat loss.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
33
PharmaCompass offers a list of Etodolac API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Etodolac manufacturer or Etodolac supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Etodolac manufacturer or Etodolac supplier.
PharmaCompass also assists you with knowing the Etodolac API Price utilized in the formulation of products. Etodolac API Price is not always fixed or binding as the Etodolac Price is obtained through a variety of data sources. The Etodolac Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Etol Fort manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Etol Fort, including repackagers and relabelers. The FDA regulates Etol Fort manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Etol Fort API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Etol Fort manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Etol Fort supplier is an individual or a company that provides Etol Fort active pharmaceutical ingredient (API) or Etol Fort finished formulations upon request. The Etol Fort suppliers may include Etol Fort API manufacturers, exporters, distributors and traders.
click here to find a list of Etol Fort suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Etol Fort DMF (Drug Master File) is a document detailing the whole manufacturing process of Etol Fort active pharmaceutical ingredient (API) in detail. Different forms of Etol Fort DMFs exist exist since differing nations have different regulations, such as Etol Fort USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Etol Fort DMF submitted to regulatory agencies in the US is known as a USDMF. Etol Fort USDMF includes data on Etol Fort's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Etol Fort USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Etol Fort suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Etol Fort Drug Master File in Japan (Etol Fort JDMF) empowers Etol Fort API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Etol Fort JDMF during the approval evaluation for pharmaceutical products. At the time of Etol Fort JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Etol Fort suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Etol Fort Drug Master File in Korea (Etol Fort KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Etol Fort. The MFDS reviews the Etol Fort KDMF as part of the drug registration process and uses the information provided in the Etol Fort KDMF to evaluate the safety and efficacy of the drug.
After submitting a Etol Fort KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Etol Fort API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Etol Fort suppliers with KDMF on PharmaCompass.
A Etol Fort CEP of the European Pharmacopoeia monograph is often referred to as a Etol Fort Certificate of Suitability (COS). The purpose of a Etol Fort CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Etol Fort EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Etol Fort to their clients by showing that a Etol Fort CEP has been issued for it. The manufacturer submits a Etol Fort CEP (COS) as part of the market authorization procedure, and it takes on the role of a Etol Fort CEP holder for the record. Additionally, the data presented in the Etol Fort CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Etol Fort DMF.
A Etol Fort CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Etol Fort CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Etol Fort suppliers with CEP (COS) on PharmaCompass.
A Etol Fort written confirmation (Etol Fort WC) is an official document issued by a regulatory agency to a Etol Fort manufacturer, verifying that the manufacturing facility of a Etol Fort active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Etol Fort APIs or Etol Fort finished pharmaceutical products to another nation, regulatory agencies frequently require a Etol Fort WC (written confirmation) as part of the regulatory process.
click here to find a list of Etol Fort suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Etol Fort as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Etol Fort API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Etol Fort as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Etol Fort and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Etol Fort NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Etol Fort suppliers with NDC on PharmaCompass.
Etol Fort Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Etol Fort GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Etol Fort GMP manufacturer or Etol Fort GMP API supplier for your needs.
A Etol Fort CoA (Certificate of Analysis) is a formal document that attests to Etol Fort's compliance with Etol Fort specifications and serves as a tool for batch-level quality control.
Etol Fort CoA mostly includes findings from lab analyses of a specific batch. For each Etol Fort CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Etol Fort may be tested according to a variety of international standards, such as European Pharmacopoeia (Etol Fort EP), Etol Fort JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Etol Fort USP).
