
Synopsis
Synopsis
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
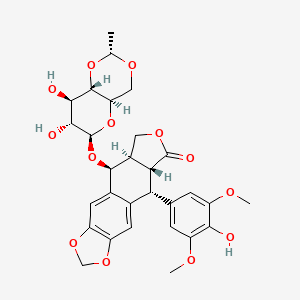

1. Alpha-d-glucopyranosyl Isomer Etoposide
2. Celltop
3. Demethyl Epipodophyllotoxin Ethylidine Glucoside
4. Eposide
5. Eposin
6. Eto Gry
7. Eto-gry
8. Etomedac
9. Etopos
10. Etoposide Pierre Fabre
11. Etoposide Teva
12. Etoposide, (5a Alpha)-isomer
13. Etoposide, (5a Alpha,9 Alpha)-isomer
14. Etoposide, (5s)-isomer
15. Etoposide, Alpha D Glucopyranosyl Isomer
16. Etoposide, Alpha-d-glucopyranosyl Isomer
17. Etoposido Ferrer Farma
18. Exitop
19. Lastet
20. Nsc 141540
21. Nsc-141540
22. Nsc141540
23. Onkoposid
24. Riboposid
25. Teva, Etoposide
26. Toposar
27. Vpside Sandoz
28. Vpside-sandoz
29. Vepesid
30. Vp 16
31. Vp 16 213
32. Vp 16-213
33. Vp 16213
34. Vp-16
35. Vp16
1. 33419-42-0
2. Vepesid
3. Toposar
4. Trans-etoposide
5. Lastet
6. (-)-etoposide
7. Vp-16
8. Zuyeyidal
9. Etoposidum
10. Etoposido
11. Etoposidum [inn-latin]
12. Vp-16-213
13. Etoposide (vp16)
14. Vp 16-213
15. Vepesid J
16. Sintopozid
17. 4-demethylepipodophyllotoxin Beta-d-ethylideneglucoside
18. Nsc-141540
19. Etoposide (vp-16)
20. Vp 16 (pharmaceutical)
21. 4'-demethylepipodophyllotoxin 9-(4,6-o-(r)-ethylidene-beta-d-glucopyranoside)
22. Vp 16
23. 6plq3cp4p3
24. Epipodophyllotoxin Vp-16213
25. Chembl44657
26. Chebi:4911
27. Nk 171
28. Demethylepipodophyllotoxin-beta-d-ethylideneglucoside
29. Nsc 141540
30. 4'-demethylepipodophyllotoxin 9-(4,6-o-ethylidene-beta-d-glucopyranoside)
31. Etosid
32. [1,3]benzodioxol-8-one
33. Etoposido [inn-spanish]
34. (5s,5ar,8ar,9r)-5-[[(2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5h-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one
35. (5s,5ar,8ar,9r)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-5-yl 4,6-o-[(1r)-ethylidene]-beta-d-glucopyranoside
36. Etopophos (phosphate Salt)
37. Etopol
38. Vp 16213
39. Nsc141540
40. 9-((4,6-o-ethylidine-beta-d-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,4-dimethyloxyphenyl)furo(3',4'':6,7)naptho-(2,3-d)-1,3-dioxol-6(5ah)-one
41. Smr000112002
42. Ccris 2392
43. Hsdb 6517
44. Vepesid (tn)
45. Einecs 251-509-1
46. Mfcd00869325
47. Unii-6plq3cp4p3
48. 4'-demethylepipodophyllotoxin Ethylidene-.beta.-d-glucoside
49. Dtxsid5023035
50. Etoposide,(s)
51. (5s,5ar,8ar,9r)-5-[[(2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5h-[2]benzofuro[5,6-f]
52. (5s,5ar,8ar,9r)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol -5-yl 4,6-o-[(1r)-ethylidene]-beta-d-glucopyranoside
53. Evp
54. Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5ah)-one, 9-[[4,6-o-(1r)-ethylidene-.beta.-d-glucopyranosyl]oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5r,5ar,8ar,9s)-
55. 4'-o-demethyl-1-o-(4,6-o-ethylidene-beta-d-glucopyranosyl)epipodophyllotoxin
56. Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-o-ethylidene-beta-d-glucopyranoside)
57. Etoposide [usan:usp:inn:ban:jan]
58. Etoposide; Vp-16
59. Cpd000112002
60. Epipodophyllotoxin-beta-d-ethyliden-glucoside, 4'-demethyl-
61. Etoposide [inn]
62. Etoposide [jan]
63. Etoposide [mi]
64. Etoposide [hsdb]
65. Etoposide [iarc]
66. Etoposide [usan]
67. Prestwick3_000396
68. Etoposide [vandf]
69. Etoposide [mart.]
70. Epipodophyllotoxin, 4'-demethyl-, 4,6-o-ethylidene-beta-d-glucopyranoside
71. Etoposide Resolution Mixture
72. Etoposide [usp-rs]
73. Etoposide [who-dd]
74. Etoposide [who-ip]
75. Schembl4259
76. Bspbio_000611
77. 9-((4,6-o-ethylidene-beta-d-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5ah)-one, (5r-(5alpha,5abeta,8aalpha,9beta(r*)))-
78. 9-((4,6-o-ethylidine-beta-d-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4- Hydroxy-3,4-dimethyloxyphenyl)furo (3',4'':6,7) Naptho-(2,3-d)-1,3-dioxol-6 (5ah)-one
79. Mls000049957
80. Mls001074951
81. Mls001424283
82. Mls002153463
83. Mls002207239
84. Mls002222184
85. Etoposide (jp17/usp/inn)
86. Bpbio1_000673
87. Gtpl6815
88. Etoposide [ep Impurity]
89. Etoposide [orange Book]
90. Etoposide [ep Monograph]
91. Etoposide [usp Impurity]
92. Etoposide [usp Monograph]
93. Etoposidum [who-ip Latin]
94. Etoposide4-o-b-d-galactopyranoside
95. Hms2052n05
96. Hms2089f14
97. Hms2096o13
98. Hms2232l03
99. Hms3713o13
100. (5r,5ar,8ar,9s)-9-(((4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-methylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8h)-one
101. Ex-a1207
102. Zinc3938684
103. Bdbm50127140
104. S1225
105. Etoposide - Cas 33419-42-0
106. Akos007930275
107. Bcp9000669
108. Ccg-101165
109. Cs-1774
110. Db00773
111. Etoposide, Synthetic, >=98%, Powder
112. Nc00415
113. Sdccgsbi-0050405.p002
114. 4'-demethyl-epipodophyllotoxin 9-[4,6-o-(r)-ethylidene-beta-d-glucopyranoside
115. Ncgc00179504-02
116. As-35312
117. Be164434
118. Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5ah)-one, 9-((4,6-o-(1r)-ethylidene-beta-d-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5r,5ar,8ar,9s)-
119. Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5ah)-one-, 9-((4,6-o-ethylidene-beta-d-glucopyranosyl)oxy)5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl), (5r-(5alpha,5abeta,8aalpha,9beta(r*)))-
120. Hy-13629
121. Etoposide Impurity C [ep Impurity]
122. Sbi-0051910.p002
123. Ab00438905
124. C01576
125. D00125
126. Ab00438905-17
127. Ab00438905-18
128. Ab00438905_19
129. 419e420
130. Q418817
131. Sr-01000763196
132. Sr-01000763196-3
133. Brd-k37798499-001-02-5
134. Brd-k37798499-001-05-8
135. Brd-k37798499-001-10-8
136. Brd-k37798499-001-14-0
137. Brd-k37798499-001-27-2
138. Etoposide, British Pharmacopoeia (bp) Reference Standard
139. Etoposide, European Pharmacopoeia (ep) Reference Standard
140. Etoposide, United States Pharmacopeia (usp) Reference Standard
141. 4''-demethylepipodophyllotoxin 9-(4,6-o-(r)-ethylidene-beta-d-glucopyranoside)
142. 4'-demethylepipodophyllotoxin 9-(4,6-o-(r)-ethylidene-.beta.-d-glucopyranoside)
143. Etoposide For System Suitability, European Pharmacopoeia (ep) Reference Standard
144. (10r,11r,15r,16s)-16-([(2r,4ar,7r,8r,8as)-7,8-dihydroxy-2-methyl-hexahydro-2h-pyrano[3,2-d][1,3]diox
145. (10r,11r,15r,16s)-16-{[(2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-methyl-hexahydro-2h-pyrano[3,2-d][1,3]dioxin-6-yl]oxy}-10-(4-hydroxy-3,5-dimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.0^{3,7}.0^{11,15}]hexadeca-1(9),2,7-trien-12-one
146. (5r,5ar,8ar,9s)-9-(((2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-methylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahy
147. (5r,5ar,8ar,9s)-9-(((2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-methylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8h)-one
148. (5r,5ar,8ar,9s)-9-((4,6-o-((1r)-ethane-1,1-diyl)-.alpha.-d-glucopyranosyl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,8,8a,9-tetrahydro(2)benzofuro(5,6-f)(1,3)benzodioxol-6(5ah)-one
149. (5r,5ar,8ar,9s)-9-[[4,6-o-(1r)-ethylidene-beta-d-glucopyranosyl]oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5ah)-one
150. (5s,5ar,8ar,9r)-5-[[(2r,4ar,6r,7r,8r,8as)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxy-phenyl)-5a,6,8a,9-tetrahydro-5h-isobenzofuro[5,6-f][1,3]benzodioxol-8-one
151. (5s,5ar,8ar,9r)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3'',4'':6,7]naphtho[2,3-d][1,3]dioxol-5-yl 4,6-o-[(1r)-ethylidene]-beta-d-glucopyranoside
152. [5r-[5?,5a?,8a?,9?(r*)]]-9-[(4,6-?-ethylidene-?-d-glucopyranosyl)oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6-(5ah)-one
153. 121471-01-0
154. 9-((4,6-o-ethylidine-beta-d-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,4-dimethyloxyphenyl)furo(3'',4'''':6,7)naptho-(2,3-d)-1,3-dioxol-6(5ah)-one
155. Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5ah)-one-, 9-((4,6-o-ethylidene-.beta.-d-glucopyranosyl)oxy)5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl), (5r-(5.alpha.,5a.beta.,8a.alpha.,9.beta.(r*)))-
| Molecular Weight | 588.6 g/mol |
|---|---|
| Molecular Formula | C29H32O13 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 5 |
| Exact Mass | 588.18429107 g/mol |
| Monoisotopic Mass | 588.18429107 g/mol |
| Topological Polar Surface Area | 161 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 969 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Etoposide |
| PubMed Health | Etoposide |
| Drug Classes | Antineoplastic Agent |
| Drug Label | TOPOSAR (etoposide injection, USP) (also commonly known as VP-16) is a semisynthetic derivative of podophyllotoxin used in the treatment of certain neoplastic diseases. It is 4'-demethylepipodophyllotoxin 9-[4,6-0-(R)-ethylidene--D-glucopyranoside].. |
| Active Ingredient | Etoposide |
| Dosage Form | Injectable; Capsule |
| Route | Injection; Oral |
| Strength | 20mg/ml; 50mg |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Accord Hlthcare; Teva Pharms Usa; Eurohlth Intl; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Etoposide |
| PubMed Health | Etoposide |
| Drug Classes | Antineoplastic Agent |
| Drug Label | TOPOSAR (etoposide injection, USP) (also commonly known as VP-16) is a semisynthetic derivative of podophyllotoxin used in the treatment of certain neoplastic diseases. It is 4'-demethylepipodophyllotoxin 9-[4,6-0-(R)-ethylidene--D-glucopyranoside].. |
| Active Ingredient | Etoposide |
| Dosage Form | Injectable; Capsule |
| Route | Injection; Oral |
| Strength | 20mg/ml; 50mg |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Accord Hlthcare; Teva Pharms Usa; Eurohlth Intl; Mylan |
Antineoplastic Agents, Phytogenic; Nucleic Acid Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Etoposide injection is indicated, in combination with other antineoplastics, for first-line treatment of testicular tumors (Evidence rating: 1A). /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1326
Etoposide is indicated in combination with other agents as first-line treatment of small cell lung carcinoma. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1326
Etoposide also is indicated, alone and in combination with other agents, for treatment of Hodgkin's and non-Hodgkin"s lymphomas and acute nonlymphocytic (myelocytic) leukemia. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1326
For more Therapeutic Uses (Complete) data for ETOPOSIDE (13 total), please visit the HSDB record page.
The major and dose-limiting adverse effect of etoposide is hematologic toxicity. Myelosuppression, which is dose related, is manifested mainly by leukopenia (principally granulocytopenia). Myelosuppression resulting in death has been reported in patients receiving etoposide. Thrombocytopenia occurs less frequently, and anemia may also occur; pancytopenia has occurred in some patients. Myelosuppression apparently is not cumulative but may be more severe in patients previously treated with other antineoplastic agents or radiation therapy. Leukopenia has reportedly occurred in 60-91% of patients receiving etoposide and was severe (leukocyte count less than 1000/cu mm) in 3-17% of patients. Neutropenia (less than 2000 cu mm) occurred in 88% of patients treated with etoposide phosphate; severe neutropenia has reportedly occurred in 22-41% of patients receiving the drug and was severe (platelet count less than 50,000/cu mm) in 1-20% of patients. Anemia has occurred in up to 33% of patients receiving etoposide. Anemia (hemoglobin less than 11 g/dL) occurred in 72% of patients treated with etoposide phosphate; severe anemia (hemoglobin less than 8 g/dL) occurred in 19% of patients treated. Granulocyte and platelet nadirs usually occur within 7-14 and 9-16 days, respectively, after administration of etoposide, and within 12-19 and 10-15 days, respectively, after administration of etoposide phosphate; leukocyte nadir has been reported to occur within 15-22 days after administration of etoposide, phosphate. Bone marrow recovery is usually complete within 20 days after administration, but may occasionally require longer periods. Fever and infection have been reported in patients with drug-induced neutropenia.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 988
Pregnancy risk category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1327
Reversible alopecia, sometimes progressing to complete baldness, has occurred in 8-66% of patients receiving etoposide. The degree of alopecia may be dose related. Stevens-Johnson syndrome has been reported infrequently in patients receiving etoposide. Rash, pigmentation, urticaria, and severe pruritus have occurred infrequently, and cutaneous radiation-recall reactions associated with etoposide have been reported. ...
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 989
Anaphylactoid reactions consisting principally of chills, rigors, diaphoresis, pruritus, loss of consciousness, nausea, vomiting, fever, bronchospasm, dyspnea, tachycardia, hypertension, and/or hypotension have occurred during or immediately after administration of etoposide or etoposide phosphate in 0.7-3% of patients receiving the drug. Other manifestations have included flushing, rash, substernal chest pain, lacrimation, sneezing, coryza, throat pain, back pain, generalized body pain, abdominal cramps, and auditory impairment.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 989
For more Drug Warnings (Complete) data for ETOPOSIDE (24 total), please visit the HSDB record page.
For use in combination with other chemotherapeutic agents in the treatment of refractory testicular tumors and as first line treatment in patients with small cell lung cancer. Also used to treat other malignancies such as lymphoma, non-lymphocytic leukemia, and glioblastoma multiforme.
FDA Label
Etoposide is an antineoplastic agent and an epipodophyllotoxin (a semisynthetic derivative of the podophyllotoxins). It inhibits DNA topoisomerase II, thereby ultimately inhibiting DNA synthesis. Etoposide is cell cycle dependent and phase specific, affecting mainly the S and G2 phases. Two different dose-dependent responses are seen. At high concentrations (10 µg/mL or more), lysis of cells entering mitosis is observed. At low concentrations (0.3 to 10 µg/mL), cells are inhibited from entering prophase. It does not interfere with microtubular assembly. The predominant macromolecular effect of etoposide appears to be the induction of DNA strand breaks by an interaction with DNA-topoisomerase II or the formation of free radicals.
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01C - Plant alkaloids and other natural products
L01CB - Podophyllotoxin derivatives
L01CB01 - Etoposide
Absorption
Absorbed well, time to peak plasma concentration is 1-1.5 hrs. Mean bioavailability is 50% (range of 25% - 75%). Cmax and AUC values for orally administered etoposide capsules display intra- and inter-subject variability. There is no evidence of first-pass effect for etoposide.
Route of Elimination
Etoposide is cleared by both renal and nonrenal processes, i.e., metabolism and biliary excretion. Glucuronide and/or sulfate conjugates of etoposide are also excreted in human urine. Biliary excretion of unchanged drug and/or metabolites is an important route of etoposide elimination as fecal recovery of radioactivity is 44% of the intravenous dose. 56% of the dose was in the urine, 45% of which was excreted as etoposide.
Volume of Distribution
The disposition of etoposide is a biphasic process with a distribution half-life of 1.5 hours. It does not cross into cerebrospinal fluid well. Volume of distribution, steady state = 18 - 29 L.
Clearance
Total body clearance = 33 - 48 mL/min [IV administration, adults]
Mean renal clearance = 7 - 10 mL/min/m^2
Excretion of etoposide in breast milk was demonstrated in a woman with acute promyelocytic leukemia receiving daily doses of 80 mg/sq m (route not stated). Peak concentrations of 0.6 to 0.8 ug/mL were measured immediately after dosing but had decreased to undetectable levels by 24 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 214 (2000)
Thirty minutes after intravenous administration of etoposide to rats, the highest concentrations were found in the liver, kidneys and small intestine. By 24 hr after the dose, the tissue concentrations were negligible.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 211 (2000)
After intravenous infusion (5 min) of etoposide phosphate to beagle dogs at doses of 57-461 mg/sq m, a dose-proportional increase was seen in the maximal plasma concentration and AUC for etoposide. The total plasma clearance rate (342-435 mL/min per sq m) and the distribution volume (22-27 L/sq m) were not dose-dependent. The peak plasma concentration occurred at the end of the infusion of etoposide phosphate, indicating rapid conversion of the pro-drug to etoposide.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 211 (2000)
Less than 4% of a dose was recovered in the bile after 48 hr in patients with biliary drainage tubes. The fecal recovery of radiolabel after intravenous administration of 3(H)etoposide (130-290 mg/sq m) was variable, representing 0-16% of dose, but the collections were known to be incomplete because of fecal retention and other difficulties associated with the poor general condition of many of the patients). In a study reported as an abstract in four patients with small-cell lung cancer given 14(C)-glucopyranoside etoposide, 56% of the radiolabel was recovered in urine and 44% in feces over five days, for a total recovery of 100 +/- 6%.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 210 (2000)
For more Absorption, Distribution and Excretion (Complete) data for ETOPOSIDE (18 total), please visit the HSDB record page.
Primarily hepatic (through O-demethylation via the CYP450 3A4 isoenzyme pathway) with 40% excreted unchanged in the urine. Etoposide also undergoes glutathione and glucuronide conjugation which are catalyzed by GSTT1/GSTP1 and UGT1A1, respectively. Prostaglandin synthases are also responsible for the conversion of etoposide to O-demethylated metabolites (quinone).
The proposed hydroxy acid metabolite of etoposide, formed by opening of the lactone ring, has been detected in human urine, but only at low concentrations, accounting for 0.2-2.2% of the administered dose.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 210 (2000)
The major urinary metabolite of etoposide in humans is reported to be the glucuronide conjugate. Although urinary glucuronide and/or sulfate conjugates were reported to account for 5-22% of an intravenous dose of etoposide, other studies suggest that the glucuronide predominates. Etoposide glucuronide in the urine of treated patients accounted for 8-17% of a dose of 0.5-3.5 g/sq m etoposide and 29% of a dose of 100-800 mg/sq m etoposide, with no other metabolites other than etoposide glucuronide detected in the latter study. In patients with renal or liver impairment given somewhat lower doses of 70-150 mg/sq m, 3-17% of the dose was excreted in the urine within 72 hr as etoposide glucuronide.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 209 (2000)
Etoposide appears to be metabolized principally at the D ring to produce the resulting hydroxy acid (probably the trans-hydroxy acid); this metabolite appears to be pharmacologically inactive. The picrolactone isomer of etoposide has been detected in two concentrations in the plasma and urine of some patients but not in others. The aglycone of etoposide and/or its conjugates have not been detected to date in patients receiving the drug. In vitro, the picrolactone isomer and aglycone of etoposide have minimal cytotoxic activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 991
Generally, few or no etoposide metabolites have been detected in plasma. Etoposide is administered as the trans-lactone, but cis-etoposide can also be detected in human urine. This might be a storage phenomenon, since isomerization sometimes occurs during freezing of plasma samples under slightly basic conditions. The cis isomer accounts for < 1% of the dose. The catechol metabolite has also been reported in patients receiving 600 mg/sq m etoposide, with an AUC of around 2.5% that of etoposide. In patients given 90 mg/sq m etoposide, the catechol metabolite represented 1.4-7.1% of the urinary etoposide and < 2% of the administered dose.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 208 (2000)
In rat liver homogenates, liver microsomes and in rats in vivo, etoposide was extensively metabolized to only one major metabolite, which was not formally identified. In perfused isolated rat liver incubated with etoposide, the total recovery in bile was 60-85%, with roughly equal amounts of etoposide and two glucuronide metabolites, confirmed as glucuronide species by liquid chromatography and mass spectrometry. After intravenous injection of 3(H)etoposide to rabbits, the total urinary excretion of radiolabel was 30% after five days, with very little thereafter. A single glucuronide metabolite was identified in rabbit urine, which was present in larger amounts than etoposide. No hydroxy acid was identified in either species.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 211 (2000)
4-11 hours
... In adults with normal renal and hepatic function, the half-life of etoposide averages 0.6-2 hours ... in the initial phase and 5.3-10.8 hours ... in the terminal phase. In one adult with impaired hepatic function, the terminal elimination half-life was reportedly 78 hours. In children with normal renal and hepatic function, the half-life of etoposide averages 0.6-1.4 hours in the initial phase and 3-5.8 hours in the terminal phase.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 991
... Elimination half-life of 3 to 7 hr in children and 4 to 8 hr in adults.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 207 (2000)
Etoposide inhibits DNA topoisomerase II, thereby inhibiting DNA re-ligation. This causes critical errors in DNA synthesis at the premitotic stage of cell division and can lead to apoptosis of the cancer cell. Etoposide is cell cycle dependent and phase specific, affecting mainly the S and G2 phases of cell division. Inhibition of the topoisomerase II alpha isoform results in the anti-tumour activity of etoposide. The drug is also capable of inhibiting the beta isoform but inhibition of this target is not associated with the anti-tumour activity. It is instead associated with the carcinogenic effect.
The drug appears to produce its cytotoxic effects by damaging DNA and thereby inhibiting or altering DNA synthesis. ... Etoposide appears to be cell-cycle dependent and cycle-phase specific, inducing G2 phase arrest and preferentially filling cells in the G2 and late S phases.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 990
Etoposide has been shown to arrest metaphase in chick fibroblasts, but its principal effect in mammalian cells appears to be in the G2 phase. At etoposide concentrations of 0.3-10 ug/ml in vitro, cells are inhibited from entering prophase; at concentrations of 10 ug/ml or higher, lysis of cells entering mitosis occurs. ... Etoposide does not inhibit microtubule assembly. Etoposide has been shown to induce single-stranded DNA breaks in HeLa cells and in murine leukemia L1210 cells in vitro; the drug also induces double-stranded DNA breaks and DNA-protein crosslinks in L1210 cells. Etoposide induced DNA damage appears to correlate well with the cytotoxicity of the drug. ... Etoposide appears to induce single-stranded DNA breaks indirectly, possibly through endonuclease activation, inhibition of intranuclear type II topoisomerase, or formation of a free-radical metabolite via an enzymatic reaction involving the hydroxyl group at the C-4' position of the E ring. Etoposide also reversibly inhibits the facilitated diffusion of nucleosides into HeLa cells in a concentration dependent manner in vitro.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 990
Etoposide may stabilize type II topoisomerase DNA complexes, preventing rejoining of single and double strand DNA breaks. Etoposide may also require cellular activation into intermediates, which then bind to DNA and disrupt cellular function.
PMID:2653712 Fleming RA et al; Clin Pharm 8 (4): 274-93 (1989)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
98
PharmaCompass offers a list of Etoposide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Etoposide manufacturer or Etoposide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Etoposide manufacturer or Etoposide supplier.
PharmaCompass also assists you with knowing the Etoposide API Price utilized in the formulation of products. Etoposide API Price is not always fixed or binding as the Etoposide Price is obtained through a variety of data sources. The Etoposide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Etoposide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Etoposide, including repackagers and relabelers. The FDA regulates Etoposide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Etoposide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Etoposide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Etoposide supplier is an individual or a company that provides Etoposide active pharmaceutical ingredient (API) or Etoposide finished formulations upon request. The Etoposide suppliers may include Etoposide API manufacturers, exporters, distributors and traders.
click here to find a list of Etoposide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Etoposide DMF (Drug Master File) is a document detailing the whole manufacturing process of Etoposide active pharmaceutical ingredient (API) in detail. Different forms of Etoposide DMFs exist exist since differing nations have different regulations, such as Etoposide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Etoposide DMF submitted to regulatory agencies in the US is known as a USDMF. Etoposide USDMF includes data on Etoposide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Etoposide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Etoposide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Etoposide Drug Master File in Japan (Etoposide JDMF) empowers Etoposide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Etoposide JDMF during the approval evaluation for pharmaceutical products. At the time of Etoposide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Etoposide suppliers with JDMF on PharmaCompass.
A Etoposide CEP of the European Pharmacopoeia monograph is often referred to as a Etoposide Certificate of Suitability (COS). The purpose of a Etoposide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Etoposide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Etoposide to their clients by showing that a Etoposide CEP has been issued for it. The manufacturer submits a Etoposide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Etoposide CEP holder for the record. Additionally, the data presented in the Etoposide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Etoposide DMF.
A Etoposide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Etoposide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Etoposide suppliers with CEP (COS) on PharmaCompass.
A Etoposide written confirmation (Etoposide WC) is an official document issued by a regulatory agency to a Etoposide manufacturer, verifying that the manufacturing facility of a Etoposide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Etoposide APIs or Etoposide finished pharmaceutical products to another nation, regulatory agencies frequently require a Etoposide WC (written confirmation) as part of the regulatory process.
click here to find a list of Etoposide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Etoposide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Etoposide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Etoposide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Etoposide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Etoposide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Etoposide suppliers with NDC on PharmaCompass.
Etoposide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Etoposide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Etoposide GMP manufacturer or Etoposide GMP API supplier for your needs.
A Etoposide CoA (Certificate of Analysis) is a formal document that attests to Etoposide's compliance with Etoposide specifications and serves as a tool for batch-level quality control.
Etoposide CoA mostly includes findings from lab analyses of a specific batch. For each Etoposide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Etoposide may be tested according to a variety of international standards, such as European Pharmacopoeia (Etoposide EP), Etoposide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Etoposide USP).
