

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Weekly News Recap #Phispers
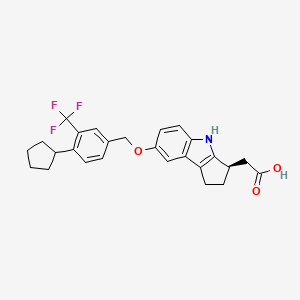

1. (r)-2-(7-((4-cyclo-pentyl-3-(trifluoromethyl)benzyl)oxy)-1,2,3,4-tetrahydro-cyclopenta(b)indol-3-yl)acetic Acid)
1. 1206123-37-6
2. Apd334
3. Apd-334
4. Etrasimod [usan]
5. 6wh8495mmh
6. Apd-334(free Acid)
7. Chembl3358920
8. Etrasimod (usan)
9. 1206123-37-6 (free Base)
10. (r)-2-(7-((4-cyclopentyl-3-(trifluoromethyl)benzyl)oxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic Acid
11. (r)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta(b)indol-3-yl) Acetic Acid
12. 2-[(3r)-7-[[4-cyclopentyl-3-(trifluoromethyl)phenyl]methoxy]-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]acetic Acid
13. Etrasimod(apd334)
14. Etrasimod [inn]
15. Etrasimod [who-dd]
16. Unii-6wh8495mmh
17. Apd334apd334
18. Gtpl9331
19. Schembl1919311
20. Bcp19558
21. Ex-a1633
22. Bdbm50041691
23. Akos032944972
24. Zinc117522788
25. Apd334-003
26. Compound 4 [pmid: 25516790]
27. Cs-6181
28. Db14766
29. Ac-35444
30. Hy-12789
31. D10930
32. F10118
33. Q27265630
34. ((3r)-7-((4-cyclopentyl-3-(trifluoromethyl)phenyl)methoxy)-1,2,3,4-tetrahydrocyclopenta(b)indol-3-yl)acetic Acid
35. (r)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic Acid
36. Cyclopent(b)indole-3-acetic Acid, 7-((4-cyclopentyl-3-(trifluoromethyl)phenyl)methoxy)-1,2,3,4-tetrahydro-, (3r)-
| Molecular Weight | 457.5 g/mol |
|---|---|
| Molecular Formula | C26H26F3NO3 |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 457.18647818 g/mol |
| Monoisotopic Mass | 457.18647818 g/mol |
| Topological Polar Surface Area | 62.3 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 695 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product ...
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
About the Company : Egis is a member of the Servier Group. Egis’ products are manufactured at 3 production sites in Hungary, which are certified by EMA,FDA, ANVISA, PMDA ,KFDA. Egis sells its produc...
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
About the Company : Specializing in natural & oncology APIs, we establish R&D and production platforms for new salt, crystal form & synthetic biology research. We cooperate with clients for IND, NDA, ...
About the Company : Curia is a global contract research, development and manufacturing organization (CDMO), offering products and services across the drug development spectrum to help our partners tur...

About the Company : Hebi Xinhe Pharmaceutical Co., Ltd. is a subsidiary of Tianjin Zhennuo Pharmaceutical Group Co., Ltd., with a registered capital of CNY 100 million. Located in Jijiashan Industrial...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Velsipity (etrasimod), an FDA approved oral, once-daily, selective sphingosine-1-phosphate (S1P) receptor modulator. Its NDA is also been accepted by Macau Special Administrative Region for adults with moderately to severely active ulcerative colitis.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: Velsipity
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Everest Medicines Announces Acceptance of VELSIPITY® New Drug Application in Macau
Details : Velsipity (etrasimod), an FDA approved oral, once-daily, selective sphingosine-1-phosphate (S1P) receptor modulator. Its NDA is also been accepted by Macau Special Administrative Region for adults with moderately to severely active ulcerative colitis.
Brand Name : Velsipity
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Velsipity (etrasimod) is a once-daily, oral, sphingosine 1-phosphate (S1P) receptor modulator that selectively binds with S1P receptor subtypes 1, 4, and 5. It is approved for the treatment of patients with moderately to severely active ulcerative colitis.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: Velsipity
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable November 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Velsipity (etrasimod) is a once-daily, oral, sphingosine 1-phosphate (S1P) receptor modulator that selectively binds with S1P receptor subtypes 1, 4, and 5. It is approved for the treatment of patients with moderately to severely active ulcerative coliti...
Brand Name : Velsipity
Molecule Type : Small molecule
Upfront Cash : Not Applicable
November 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Velsipity (etrasimod), an oral, once-daily, selective sphingosine-1-phosphate (S1P) receptor modulator, is approved by the FDA for adults with moderately to severely active ulcerative colitis (UC).
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: Velsipity
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
U.S. FDA Approves Pfizer’s VELSIPITY™ for Adults with Moderately to Severely Active Ulcerative...
Details : Velsipity (etrasimod), an oral, once-daily, selective sphingosine-1-phosphate (S1P) receptor modulator, is approved by the FDA for adults with moderately to severely active ulcerative colitis (UC).
Brand Name : Velsipity
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
APD334 (etrasimod) is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator designed for optimized pharmacology and engagement of S1P receptors 1, 4, and 5. It is being investigated for the treatment of moderate-severe active ulcerative colitis.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: APD334
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Pfizer Inc
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Pfizer Inc
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : APD334 (etrasimod) is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator designed for optimized pharmacology and engagement of S1P receptors 1, 4, and 5. It is being investigated for the treatment of moderate-severe active ul...
Brand Name : APD334
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Etrasimod is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator designed for optimized pharmacology and engagement of S1P receptors 1, 4, and 5. In addition to UC, it is being investigated for a range of other immuno-inflammatory diseases.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: APD334
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Everest Medicines
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 21, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Everest Medicines
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Etrasimod is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator designed for optimized pharmacology and engagement of S1P receptors 1, 4, and 5. In addition to UC, it is being investigated for a range of other immuno-inflamma...
Brand Name : APD334
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 21, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
APD334 (Etrasimod) is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator designed for optimized pharmacology and engagement of S1P receptors 1, 4, and 5.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: APD334
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Pfizer Presents ELEVATE Pivotal Findings Demonstrating Etrasimod’s Potentially Best-in-Class Pro...
Details : APD334 (Etrasimod) is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator designed for optimized pharmacology and engagement of S1P receptors 1, 4, and 5.
Brand Name : APD334
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Etrasimod is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator designed for optimized pharmacology and engagement of S1P receptors 1, 4, and 5.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: APD334
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Pfizer Inc
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 29, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Pfizer Inc
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Etrasimod is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator designed for optimized pharmacology and engagement of S1P receptors 1, 4, and 5.
Brand Name : APD334
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 29, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Etrasimod is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator being investigated for a range of immuno-inflammatory diseases including ulcerative colitis, Crohn’s Disease, atopic dermatitis, eosinophilic esophagitis, and alopecia areata.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: APD334
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 23, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Etrasimod is an oral, once-a-day, selective sphingosine 1-phosphate (S1P) receptor modulator being investigated for a range of immuno-inflammatory diseases including ulcerative colitis, Crohn’s Disease, atopic dermatitis, eosinophilic esophagitis, and ...
Brand Name : APD334
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 23, 2022

Details:
Arena Pharmaceuticals brings to Pfizer a portfolio of diverse and promising development-stage therapeutic candidates in gastroenterology, dermatology, and cardiology, including etrasimod, an oral, selective sphingosine 1-phosphate (S1P) receptor modulator.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: APD334
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Pfizer Inc
Deal Size: $6,700.0 million Upfront Cash: $6,700.0 million
Deal Type: Acquisition March 11, 2022

Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Pfizer Inc
Deal Size : $6,700.0 million
Deal Type : Acquisition
Pfizer Completes Acquisition of Arena Pharmaceuticals
Details : Arena Pharmaceuticals brings to Pfizer a portfolio of diverse and promising development-stage therapeutic candidates in gastroenterology, dermatology, and cardiology, including etrasimod, an oral, selective sphingosine 1-phosphate (S1P) receptor modulato...
Brand Name : APD334
Molecule Type : Small molecule
Upfront Cash : $6,700.0 million
March 11, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the terms of the agreement, Pfizer will acquire all the outstanding shares of Arena and it's pipeline including lead product etrasimod, an oral, selective sphingosine 1-phosphate receptor modulator currently in development for a range of immuno-inflammatory diseases.
Lead Product(s): Etrasimod
Therapeutic Area: Gastroenterology Brand Name: APD334
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Pfizer Inc
Deal Size: $6,700.0 million Upfront Cash: $6,700.0 million
Deal Type: Acquisition December 13, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Etrasimod
Therapeutic Area : Gastroenterology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Pfizer Inc
Deal Size : $6,700.0 million
Deal Type : Acquisition
Pfizer to Acquire Arena Pharmaceuticals
Details : Under the terms of the agreement, Pfizer will acquire all the outstanding shares of Arena and it's pipeline including lead product etrasimod, an oral, selective sphingosine 1-phosphate receptor modulator currently in development for a range of immuno-inf...
Brand Name : APD334
Molecule Type : Small molecule
Upfront Cash : $6,700.0 million
December 13, 2021

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]https://www.pharmacompass.com/radio-compass-blog/fda-approvals-rise-49-in-2023-crispr-s-gene-editing-therapy-sees-light-of-day
Global Sales Information

Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
