

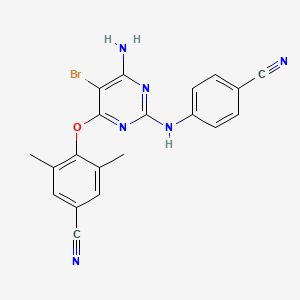

1. 4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)-4-pyrimidinyl)oxy)-3,5-dimethyl-benzonitrile
2. Benzonitrile, 4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)-4-pyrimidinyl)oxy)-3,5-dimethyl-
3. Intelence
4. R165335
5. Tmc 125
6. Tmc-125
7. Tmc125 Cpd
1. 269055-15-4
2. Intelence
3. Tmc-125
4. Tmc125
5. Etravirine (tmc125)
6. Tmc 125
7. R165335
8. R-165335
9. 4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile
10. 4-({6-amino-5-bromo-2-[(4-cyanophenyl)amino]pyrimidin-4-yl}oxy)-3,5-dimethylbenzonitrile
11. 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethylbenzonitrile
12. 0c50hw4fo1
13. Chembl308954
14. Chebi:63589
15. Etravirine-d8
16. 4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)-4-pyrimidinyl)oxy)-3,5-dimethyl-benzonitrile
17. 4-(6-amino-5-bromo-2-(4-cyanoanilino)pyrimidin-4-yloxy)-3,5-dimethylbenzonitrile
18. Benzonitrile, 4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)-4-pyrimidinyl)oxy)-3,5-dimethyl-
19. Benzonitrile, 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethyl-
20. Benzonitrile,4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethyl-
21. 65b
22. Intelence(tm)
23. Dapy Deriv
24. 4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin-4-yloxy)-3,5-dimethylbenzonitrile
25. Intelence (tn)
26. Diaminopyrimidine Deriv
27. Tmc125 Cpd
28. Unii-0c50hw4fo1
29. Etravirine (jan/usan/inn)
30. Etravirine [usan:inn:ban:jan]
31. Etravirine [inn]
32. Etravirine- Bio-x
33. R 165335
34. Etravine; Etravirine
35. 4-[6-amino-5-bromo-2-(4-cyanoanilino)pyrimidin-4-yl]oxy-3,5-dimethylbenzonitrile
36. R165335-tmc125
37. 3m8p
38. Etravirine [mi]
39. Tmc-125/r-165335
40. Etravirine [jan]
41. Etravirine [usan]
42. Etravirine [vandf]
43. Etravirine [mart.]
44. Etravirine [who-dd]
45. Schembl52691
46. Etravirine [ema Epar]
47. Etravirine [orange Book]
48. Dtxsid30181412
49. Hms3651p20
50. Zinc602632
51. Bcp03562
52. Ex-a4282
53. Bdbm50103642
54. S3080
55. 4-({6-amino-5-bromo-2-[(4-
56. Akos015896355
57. Ac-8503
58. Bcp9000006
59. Ccg-269074
60. Cs-0435
61. Db06414
62. He-0088
63. Pb32778
64. 4-[[4-amino-5-bromo-6-(4-cyano-2,6-dimethylphenyloxy)-2-pyrimidinyl]amino]benzonitrile
65. 4-[6-amino-5-bromo-2-(4-cyanoanilino)pyrimidin-4-yl]oxy-3,5-dimethyl-benzonitrile
66. Ncgc00345885-02
67. Ncgc00345885-04
68. Ba164437
69. Hy-90005
70. Bcp0726000193
71. Db-067700
72. Am20080899
73. Cyanophenyl)amino]pyrimidin-4-yl}oxy)-3,5-
74. Ft-0658058
75. Ft-0668442
76. Ft-0668443
77. Sw219570-1
78. A26965
79. D04112
80. Ab01566873_01
81. A818671
82. Q414762
83. Sr-01000944895
84. J-513179
85. Sr-01000944895-1
86. 4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin-4-ylamino)-3,5-dimethylbenzonitrile
87. 4-[[4-amino 5-bromo-6-(4-cyano-2,6-dimethylphenyloxy)-2-pyrimidinyl]amino]benzonitrile
88. 4-[[4-amino-5-bromo-6-(4-cyano-2,6-dimethylphenyloxy)-2-pyrimidinyl]-amino]benzonitrile
89. 4-[[6-amino-5-bromo-2-(4-cyanoanilino)-4-pyrimidinyl]oxy]-3,5-dimethylbenzonitrile
90. 4-[6-amino-5-bromo-2-(4-cyano-phenylamino)-pyrimidin-4-yloxy]-3,5-dimethyl-benzonitrile
91. 4-((6-amino-5-bromo-2-((4-cyanophenyl) Amino) Pyrimidin-4-yl) Oxy)-3, 5-dimethylbenzonitrile
92. 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3, 5 Cdimethylbenzonitrile
93. 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethyl -benzonitrile
94. 4-[6-azanyl-5-bromanyl-2-[(4-cyanophenyl)amino]pyrimidin-4-yl]oxy-3,5-dimethyl-benzenecarbonitrile
| Molecular Weight | 435.3 g/mol |
|---|---|
| Molecular Formula | C20H15BrN6O |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 434.04907 g/mol |
| Monoisotopic Mass | 434.04907 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 609 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Intelence |
| PubMed Health | Etravirine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | INTELENCE (etravirine) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) of human immunodeficiency virus type 1 (HIV-1).The chemical name for etravirine is 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethylbenzon... |
| Active Ingredient | Etravirine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 25mg; 100mg |
| Market Status | Prescription |
| Company | Janssen R And D |
| 2 of 2 | |
|---|---|
| Drug Name | Intelence |
| PubMed Health | Etravirine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | INTELENCE (etravirine) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) of human immunodeficiency virus type 1 (HIV-1).The chemical name for etravirine is 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethylbenzon... |
| Active Ingredient | Etravirine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 25mg; 100mg |
| Market Status | Prescription |
| Company | Janssen R And D |
Indicated as an adjunct therapy in the treatment of adult HIV-1 infections resistant to therapy with other NNRTIs and antiretroviral agents.
FDA Label
Intelence, in combination with a boosted protease inhibitor and other antiretroviral medicinal products, is indicated for the treatment of human-immunodeficiency-virus-type-1 (HIV-1) infection in antiretroviral-treatment-experienced adult patients and in antiretroviral-treatment-experienced paediatric patients from six years of age.
This indication is based on week-48 analyses from two phase-III trials in highly pretreated patients where Intelence was investigated in combination with an optimised background regimen (OBR) which included darunavir/ritonavir. The indication in paediatric patients is based on 48-week analyses of a single-arm, phase-II trial in antiretroviral-treatment-experienced paediatric patients.
Clinical trials have shown no prolongation of QT intervals on electrocardiograms after 8 days of dosing.
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J05AG04
J05AG04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AG - Non-nucleoside reverse transcriptase inhibitors
J05AG04 - Etravirine
Absorption
Maximum oral absorption is achieved in 2.5-4 hours. Absorption is unaffected by the concomitant use of oral ranitidine or omeprazole, which decrease gastric acidity. Administration under fasting conditions resulted in a near 50% decrease in systemic exposure (AUC) when compared to administration after a meal.
Route of Elimination
After a 800mg dose of radio-labelled etraverine, 93.7% was found to undergo fecal elimination, with 81.2% - 86.4% eliminated unchanged. 1.2% of the dose was renally eliminated, changed. Etravirine is dialyzable (hemodialysis).
Volume of Distribution
Distribution of etravirine into compartments other than plasma has not been evaluated in humans.
Clearance
Renal clearance of etravirine is negligible (<1.2%), thus no dose adjustments are required in patients with renal impairment. Clearance is shown to be reduced in patients with Hepatitis B and/or co-infection, however, the safety profile of etravirine does not call for dosage adjustments.
Metabolized (in vitro) by the liver CYP450 enzymes: CYP3A4, CYP2C9, CYP2C19. The major metabolites formed by a methyl hydroxylation of the dimethylbenzonitrile moiety retained less than 90% of etravirine's activity.
Half life of 9.05-41 hours.
Etravirine exerts its effects via direct inhibition of the reverse transcriptase enzyme of human immunodeficiency virus type 1 (HIV-1). It directly binds reverse transcriptase and consequently blocks DNA-dependent and RNA-dependent polymerase activity. Etravirine does not inhibit human DNA polymerase alpha, beta or gamma.

LOOKING FOR A SUPPLIER?