
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF
0
FDA Orange Book

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
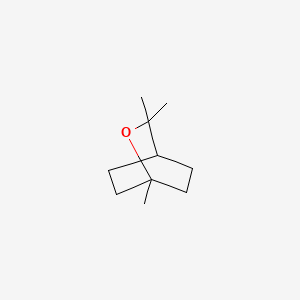

1. 1,8 Cineol
2. 1,8 Cineole
3. 1,8 Epoxy P Menthane
4. 1,8-cineol
5. 1,8-cineole
6. 1,8-epoxy-p-menthane
7. Cineole
8. Soledum
1. Cineole
2. 1,8-cineole
3. 470-82-6
4. 1,8-cineol
5. Cajeputol
6. 1,8-epoxy-p-menthane
7. Eucalyptole
8. Eucapur
9. Zineol
10. Terpan
11. P-cineole
12. 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane
13. Eukalyptol
14. 1,8-oxido-p-menthane
15. Eucalyptus Oil
16. Cineol
17. Cucalyptol
18. Soledum
19. P-menthane, 1,8-epoxy-
20. Eukalyptol [czech]
21. Eucalyptol (natural)
22. Fema No. 2465
23. Zedoary Oil
24. 2-oxabicyclo[2.2.2]octane, 1,3,3-trimethyl-
25. 8000-48-4
26. Cineole (van)
27. Nci-c56575
28. 2-oxabicyclo(2.2.2)octane, 1,3,3-trimethyl-
29. Eucaly
30. 1,3,3-trimethyl-2-oxabicyclo(2.2.2)octane
31. 2-oxa-1,3,3-trimethylbicyclo(2.2.2)octane
32. Nsc 6171
33. Nsc-6171
34. Nsc6171
35. 2,2,4-trimethyl-3-oxabicyclo[2.2.2]octane
36. 2-oxa-1,3,3-trimethylbicyclo[2.2.2]octane
37. Rv6j6604tk
38. Cnl
39. 4,7,7-trimethyl-8-oxabicyclo[2.2.2]octane
40. Chebi:27961
41. Eucalyptol [usan]
42. Ncgc00091666-01
43. Ncgc00091666-04
44. Dsstox_cid_616
45. Dsstox_rid_75692
46. Dsstox_gsid_20616
47. (1s,4s)-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane
48. Eucalyptus Citriodora Oil
49. Eucalyptol 1000 Microg/ml In Methanol
50. Unii-rv6j6604tk
51. Cas-470-82-6
52. Smr000471853
53. Ccris 3727
54. Hsdb 991
55. Eucalyptol [usan:usp]
56. Einecs 207-431-5
57. Mfcd00167977
58. Terpane
59. Cyneol
60. Bidd:er0481
61. Ai3-00578
62. Eucalyptol,(s)
63. Eucalyptol (usp)
64. 1.8-cineole
65. Eucalyptol, 99%
66. Eucalyptol, Ph Helv
67. P-menthane,8-epoxy-
68. Cineole (1,8-)
69. Eucalyptol [ii]
70. Eucalyptol [mi]
71. Wln: T66 A B Aotj B1 B1 F1
72. Cineole [inci]
73. Eucalyptol [fcc]
74. 1,8-cineol-[d3]
75. Cineole [mart.]
76. Spectrum2_000221
77. Spectrum3_000683
78. Spectrum4_001747
79. Spectrum5_000704
80. Eucalyptol [fhfi]
81. Eucalyptol [hpus]
82. Eucalyptol [hsdb]
83. Eucalyptol [inci]
84. Cineole [who-dd]
85. Eucalyptol [vandf]
86. Bmse000523
87. Ec 207-431-5
88. Eucalyptol [usp-rs]
89. Schembl19622
90. Schembl41020
91. Bspbio_002405
92. Kbiogr_002194
93. Mls001050089
94. Mls001066338
95. Divk1c_000333
96. Spectrum1500294
97. Spbio_000261
98. Cineole [ep Monograph]
99. Eucalyptol, Analytical Standard
100. Chembl485259
101. Gtpl2464
102. Chembl1231862
103. Chembl1397305
104. Dtxsid4020616
105. Schembl13554591
106. Schembl17836873
107. Hms501a15
108. Kbio1_000333
109. Kbio3_001625
110. Eucalyptol [usp Impurity]
111. Ninds_000333
112. Eucalyptol [usp Monograph]
113. Hms2271p04
114. Pharmakon1600-01500294
115. Zinc967566
116. Hy-n0066
117. Tox21_111161
118. Tox21_202090
119. Tox21_302902
120. Bdbm50459887
121. Ccg-36080
122. Nsc760388
123. Acylated Oxime Isatin Derivative, 19
124. Akos015903223
125. Akos016034339
126. Akos037514637
127. Tox21_111161_1
128. Ccg-266254
129. Cs-8146
130. Db03852
131. Lmpr0102090019
132. Nsc-760388
133. Idi1_000333
134. Eucalyptol, Tested According To Ph.eur.
135. Ncgc00091666-02
136. Ncgc00091666-03
137. Ncgc00091666-05
138. Ncgc00095774-01
139. Ncgc00178671-01
140. Ncgc00256479-01
141. Ncgc00259639-01
142. Ac-20234
143. Eucalyptol, Natural, >=99%, Fcc, Fg
144. Ls-13868
145. Nci60_005108
146. 1,3-trimethyl-2-oxabicyclo[2.2.2]octane
147. 2-oxa-1,3-trimethylbicyclo[2.2.2]octane
148. Db-070775
149. 2-oxabicyclo[2.2.2]octane,3,3-trimethyl-
150. Ft-0607033
151. Ft-0626369
152. 1,3,3-trimethyl-2-oxabicyclo[2,2,2]octane
153. A15662
154. C09844
155. D04115
156. Ab01563262_01
157. Q161572
158. Sr-01000763816
159. Sr-01000763816-2
160. W-106080
161. 1,8-cineole, Primary Pharmaceutical Reference Standard
162. Cineole, European Pharmacopoeia (ep) Reference Standard
163. Eucalyptol, Certified Reference Material, Tracecert(r)
164. F0001-1260
165. Eucalyptol, United States Pharmacopeia (usp) Reference Standard
166. Eucalyptol (cineole), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 154.25 g/mol |
|---|---|
| Molecular Formula | C10H18O |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 154.135765193 g/mol |
| Monoisotopic Mass | 154.135765193 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 164 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Cineole has mucolytic, bronchodilating and anti-inflammatory properties and reduces the exacerbation rate in patients suffering from COPD, as well as ameliorates symptoms in patients suffering from asthma and rhinosinusitis. Based on these effects, we therefore postulated the hypothesis that patients with acute bronchitis would also benefit from therapy with Cineole. As part of a double-blind, placebo-controlled, multi-center-study, a total of 242 patients with confirmed acute bronchitis was randomly selected to participate. Over a period of 10 days, all patients were administered 3 x 200 mg of Cineole, or a respective placebo, per day. The primary outcome measure was a Bronchitis Sum Score, which summarizes the relevant symptoms of acute bronchitis. After 4 days of treatment it was notable, that the patient group treated with Cineole, showed significantly more improvements of the bronchitis-sum-score than those of the placebo group (p?=?0.0383). The statistical significant difference of the individual outcome measures was especially underlined by the frequency of cough fits by p?=?0.0001 after 4 days. The effects of Cineole in the treatment of acute bronchitis were clearly measurable and could be proven after a treatment period of merely 4 days. This study corroborates the fact that Cineole actively and significantly reduces cough frequency after four days. ...
PMID:24261680 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3842692 Fischer J, Dethlefsen U; Cough 9 (1): 25 (2013)
/EXPL THER/ The clinical effects of mucolytics in patients with chronic obstructive pulmonary disease (COPD) are discussed controversially. Cineole is the main constituent of eucalyptus oil and mainly used in inflammatory airway diseases as a mucolytic agent. We hypothesized that its known mucolytic, bronchodilating and anti-inflammatory effects as concomitant therapy would reduce the exacerbation rate and show benefits on pulmonary function tests as well as quality of life in patients with COPD. In this double-blind, placebo-controlled multi-center-study we randomly assigned 242 patients with stable COPD to receive 200 mg of cineole or placebo 3 times daily as concomitant therapy for 6 months during winter-time. The frequency, duration and severity of exacerbations were combined as primary outcome measures for testing as multiple criteria. Secondary outcome measures included changes of lung function, respiratory symptoms and quality of life as well as the single parameters of the exacerbations. Baseline demographics, lung function and standard medication of both groups were comparable. During the treatment period of 6 months the multiple criteria frequency, severity and duration of exacerbations were significantly lower in the group treated with cineole in comparison to placebo. Secondary outcome measures validated these findings. Improvement of lung function, dyspnea and quality of life as multiple criteria were statistically significant relative to placebo. Adverse events were comparable in both groups. Concomitant therapy with cineole reduces exacerbations as well as dyspnea and improves lung function and health status. This study further suggests cineole as an active controller of airway inflammation in COPD by intervening in the pathophysiology of airway inflammation of the mucus membrane.
PMID:19624838 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2720945 Worth H et al; Respir Res 10: 69 (2009)
/EXPL THER/ The clinical effects of mucolytics in patients with chronic obstructive pulmonary disease (COPD) are discussed controversially. Cineole is the main constituent of eucalyptus oil and mainly used in inflammatory airway diseases as a mucolytic agent. We hypothesized that its known mucolytic, bronchodilating and anti-inflammatory effects as concomitant therapy would reduce the exacerbation rate and show benefits on pulmonary function tests as well as quality of life in patients with COPD. In this double-blind, placebo-controlled multi-center-study we randomly assigned 242 patients with stable COPD to receive 200 mg of cineole or placebo 3 times daily as concomitant therapy for 6 months during winter-time. The frequency, duration and severity of exacerbations were combined as primary outcome measures for testing as multiple criteria. Secondary outcome measures included changes of lung function, respiratory symptoms and quality of life as well as the single parameters of the exacerbations. Baseline demographics, lung function and standard medication of both groups were comparable. During the treatment period of 6 months the multiple criteria frequency, severity and duration of exacerbations were significantly lower in the group treated with cineole in comparison to placebo. Secondary outcome measures validated these findings. Improvement of lung function, dyspnea and quality of life as multiple criteria were statistically significant relative to placebo. Adverse events were comparable in both groups. Concomitant therapy with cineole reduces exacerbations as well as dyspnea and improves lung function and health status. This study further suggests cineole as an active controller of airway inflammation in COPD by intervening in the pathophysiology of airway inflammation of the mucus membrane.
PMID:19624838 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2720945 Worth H et al; Respir Res 10: 69 (2009)
As an active agent, eucalyptus oil has been indicated for relief of the symptoms of catarrhal colds, and/or the relief of the symptoms of minor muscular sprains and cramps.
Lipophilic monoterpene formulations of eucalyptus oil appear to be readily absorbed orally, with a primarily oxidative metabolism that might necessitate induction of the cytochrome P450 enzyme system and subsequent urinary excretion. Gastrointestinal absorption of eucalyptus appears to be rapid and may be enhanced by the intake of lipids and milk. 1,8-cineole (which makes up to as much as 90% of most commonly used cineole-based eucalyptus oils) has also been found in vitro and in animals to possess cytochrome P450 inducing activity.
Flavoring Agents
Substances added to foods and medicine to improve the taste. (See all compounds classified as Flavoring Agents.)
Insect Repellents
Substances causing insects to turn away from them or reject them as food. (See all compounds classified as Insect Repellents.)
Solvents
Liquids that dissolve other substances (solutes), generally solids, without any change in chemical composition, as, water containing sugar. (Grant and Hackh's Chemical Dictionary, 5th ed) (See all compounds classified as Solvents.)
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Mouthwashes
Solutions for rinsing the mouth, possessing cleansing, germicidal, or palliative properties. (From Boucher's Clinical Dental Terminology, 4th ed) (See all compounds classified as Mouthwashes.)
R - Respiratory system
R05 - Cough and cold preparations
R05C - Expectorants, excl. combinations with cough suppressants
R05CA - Expectorants
R05CA13 - Cineole
Absorption
Common monoterpenoid compound preparations of eucalyptus oil have been observed to be readily absorbed after dermal application, likely due to their lipophilic character. Although maximal plasma levels were demonstrated in as short a time period as 10 minutes even with thicker preparations like eucalyptus oil ointments, like many other topically applied agents, the extent of absorption is also likely largely dependent upon additional factors like the size of treated skin area, patient skin condition(s), concentrations of the applied substance, and time of exposure to the substance. Currently, more data regarding the oral absorption of eucalyptus would be useful, given the relative lack of existing information. Lipophilic monoterpene compound formulations of eucalyptus oil seems to be readily absorbed orally. Regardless, there is some data that suggests that the upper part of the gastrointestinal tract has no particularly significant role in the absorption of cineole based eucalyptus oil. Pulmonary absorption of eucalyptus oil is also possible although little information exists regarding this element at the moment. Nevertheless, 1,8-cineol (which makes up to as much as 90% of most commonly used cineole-based eucalyptus oils) appears to be well absorbed via inhalation with peak plasma levels observed reportedly at 18 minutes. Given the three main constituents from Eucalyptus globulus Labill fruits, the intestinal absorption of macrocarpal A (M-A), macrocarpal B (M-B), and cypellocarpa C (Cy-C) is predominantly via passive diffusion while Cy-C demonstrates some partly ATP-dependent absorption.
Route of Elimination
Studies suggest the route of elimination for cineole or eucalyptol (which makes up to as much as 90% of most commonly used cineole-based eucalyptus oils) in brushtail possum (Trichosurus vulpecula), rats, and rabbit subjects as being in the urine.
Volume of Distribution
Studies have determined a large terminal volume of distribution for cineole or eucalyptol (which makes up to as much as 90% of most commonly used cineole-based eucalyptus oils) of 27 l/kg in brushtail possum (Trichosurus vulpecula).
Clearance
Studies have determined a high clearance rate for cineole or eucalyptol (which makes up to as much as 90% of most commonly used cineole-based eucalyptus oils) of 43 ml/min/kg in brushtail possum (Trichosurus vulpecula).
With in vivo models, eucalyptol or cineole (which make up to as much as 90% of most commonly used cineole-based eucalyptus oils), undergoes oxidation to form hydroxycineole which is excreted as glucuronide. In rats, 2-hydroxycineole, 3-hydroxycineole, and 1,8--dihydroxycineol-9-oic acid were identified as main urinary metabolites. After oral administration to brushtail possums, p-cresol, 9-hydroxycineole, and Cineole-9-oic acid were found in urine. Rabbits given eucalyptol by savage excreted 2-exo- and 2-endo-hydroxycineole in the urine. The monterpene bicyclic ketone verbenone is a known component in eucalyptus globules. In one study, this component was observed to be converted to 10-hydroxyverbenone by rat and human liver microsomal cytochrome P450 enzymes, and indicated that CYP2A6 is a principal enzyme in verbenone hydroxylation in humans.
The biotransformation of 1,8-cineole was investigated using human liver microsomes. The metabolite was established as 2-exo-hydroxy-1,8-cineole. 1,8-cineole 2-hydroxylation catalyzed by human liver microsomes was found to be efficiently conducted.
European Chemicals Agency (ECHA); Registered substances, Cineole (CAS Number: 470-82-6) (EC Number: 207-431-5) (Last modified: January 19, 2015). Available from, as of June 22, 2015: https://echa.europa.eu/
Cineole was administered to male rats once daily by gastric intubation for 20 days. Urine was collected and major metabolites identified as methyl ester of 1,8-dihydroxy-10-carboxy p-methane, 2-hydroxy cineole and 3-hydroxy cineole.
European Chemicals Agency (ECHA); Registered substances, Cineole (CAS Number: 470-82-6) (EC Number: 207-431-5) (Last modified: January 19, 2015). Available from, as of June 22, 2015: https://echa.europa.eu/
Nature of non-conjugated metabolites of 1,8-cineole in urine and feces of brushtail possum yield p-cresol, 9-hydroxycineole and cineol-9-oic acid.
Southwell IA et al; Xenobiotica 10(1) 17 (1980)
1,8-Cineole has known human metabolites that include 2alpha-hydroxy-1,8-cineole and 3alpha-hydroxy-1,8-cineole.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Studies have determined a terminal half-life for cineole or eucalyptol (which makes up to as much as 90% of most commonly used cineole-based eucalyptus oils) of approximately 7h in brushtail possum (Trichosurus vulpecula).
The general consensus is that the exact mechanism of action of eucalyptus oil is largely unknown at this time but comprises various hypotheses from various studies. Cineol containing preparations of eucalyptus oil may contain up to 80% (or more) 1,8-cineole and is one of the most common types of eucalyptus oil formulations used. As an active agent indicated for relieving certain cold symptoms and/or certain muscular sprains and cramps, it is believed that eucalyptus oil may possess some antimicrobial and anti-inflammatory activities. Some in vitro studies of human blood monocytes suggest a dose-dependent effect of eucalyptus oil to elicit significant inhibition of multiple cytokines, perhaps in the treatment of airway inflammation. Moreover, other studies in animal models discuss the possibility of eucalyptus oil demonstrating anti-inflammatory and anti-nociceptive effects that potentially account for inhibiting the formation of prostaglandins and cytokines by stimulated monocytes in vitro. Furthermore, additional studies have observed eucalyptus oil anti-viral activity against herpes simplex virus (HSV-1, HSV-2) in cell cultures as well as the demonstration of broad antimicrobial activity of eucalyptus medicinal plant extracts against Alicyclobacillus acidoterretris, Bacillus cereus, E. coli, Enterococcus faecalis, MRSA, Propionibacterium acnes, S. aureus, fungus including C. albicans isolates, Trichophyton mentagrophytes, and other Gram-positive bacteria. Specific activity against periodontopathic bacteria, such as Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, Streptococcus mutans, and Streptococcus sobrinus has also been observed.

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?