
Synopsis
Synopsis
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
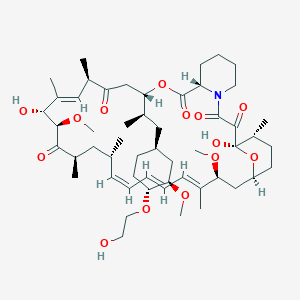

1. 001, Rad
2. 40-o-(2-hydroxyethyl)-rapamycin
3. Afinitor
4. Certican
5. Rad 001
6. Rad, Sdz
7. Rad001
8. Sdz Rad
9. Sdz-rad
10. Zortress
1. 001, Rad
2. 40-o-(2-hydroxyethyl)-rapamycin
3. 40-o-(2-hydroxyethyl)rapamycin
4. Afinitor
5. Certican
6. Rad
7. Rad 001
8. Rad, Sdz
9. Rad001
10. Sdz Rad
11. Sdz-rad
12. Zortress
13. 159351-69-6
14. Votubia
15. 42-o-(2-hydroxyethyl)rapamycin
16. Rad-001
17. Afinitor Disperz
18. Chebi:68478
19. Rapamycin, 42-o-(2-hydroxyethyl)-
20. 9hw64q8g6g
21. Rad 666
22. Rad-666
23. Everolimus (inn)
24. Ncgc00167512-01
25. Everolimus (rad001)
26. Everolimus [inn]
27. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,27-dihydroxy-3-{(2r)-1-[(1s,3r,4r)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3h-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(4h,6h,31h)-pentone
28. (1r,9s,12s,15r,16e,18r,19r,21r,23s,24e,26e,28e,30s,35r)-1,18-dihydroxy-12-{(2r)-1-[(1s,3r,4r)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.0(4,9)]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone
29. Everolimus [usan]
30. Everolimusum
31. Nsc733504
32. Everolimus Solution
33. Sdzrad
34. Everolimus Solution, 1.0 Mg/ml In Acetonitrile
35. Xience V
36. Everolimus [mi]
37. Everolimus - Rad001
38. Everolimus [jan]
39. Everolimus [vandf]
40. Everolimus [mart.]
41. Schembl4378
42. Dsstox_cid_20599
43. Dsstox_rid_79508
44. Everolimus [usp-rs]
45. Everolimus [who-dd]
46. Nvp-rad001
47. Unii-9hw64q8g6g
48. Dsstox_gsid_40599
49. Everolimus [ema Epar]
50. Everolimus [usan:inn:ban]
51. Nvp-rad-001
52. Everolimus, Analytical Standard
53. Gtpl5889
54. Rad-001c
55. Chembl1908360
56. Dtxsid0040599
57. Everolimus [orange Book]
58. Hsdb 8255
59. Everolimus [ep Monograph]
60. Everolimus; Rad001; Sdz-rad
61. 42-o-(2-hydroxyethyl)-rapamycin
62. Ex-a2057
63. Tox21_112510
64. Bdbm50088378
65. Akos015850977
66. Zinc169677008
67. Cs-0064
68. Db01590
69. (1r,9s,12s,15r,16e,18r,19r,21r,23s,24e,26e,28e,30s,32s,35r)-1,18-dihydroxy-12-((1r)-2-((1s,3r,4r)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl)-1-methylethyl)-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo(30.3.1.0(sup 4,9))hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone
70. (1r,9s,12s,15r,16e,18r,19r,21r,23s,24e,26e,28e,30s,32s,35r)-1,18-dihydroxy-12-((1r)-2-((1s,3r,4r)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl)-1-methylethyl)-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo(30.3.1.04,9)hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone
71. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3-((1r)-2-((1s,3r,4r)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl)-1-methylethyl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3h-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,28,29(4h,6h,31h)-pentone
72. As-16971
73. Hy-10218
74. Cas-159351-69-6
75. 351e696
76. Q421052
77. Q-101413
78. Brd-k13514097-001-01-2
79. Brd-k13514097-001-05-3
80. Dihydroxy-[(1r)-2-[(1s,3r,4r)-4-(2-hydroxyethoxy)-3-methoxy-cyclohexyl]-1-methyl-ethyl]-dimethoxy-hexamethyl-[?]pentone
81. Everolimus Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 958.2 g/mol |
|---|---|
| Molecular Formula | C53H83NO14 |
| XLogP3 | 5.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 9 |
| Exact Mass | 957.58135632 g/mol |
| Monoisotopic Mass | 957.58135632 g/mol |
| Topological Polar Surface Area | 205 Ų |
| Heavy Atom Count | 68 |
| Formal Charge | 0 |
| Complexity | 1810 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 15 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Afinitor |
| PubMed Health | Everolimus (By mouth) |
| Drug Classes | Antineoplastic Agent, Immune Suppressant |
| Drug Label | AFINITOR (everolimus), an inhibitor of mammalian target of rapamycin (mTOR), is an antineoplastic agent.The chemical name of everolimus is (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18- dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxy... |
| Active Ingredient | Everolimus |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 5mg; 7.5mg; 10mg |
| Market Status | Prescription |
| Company | Novartis |
| 2 of 4 | |
|---|---|
| Drug Name | Zortress |
| PubMed Health | Everolimus (By mouth) |
| Drug Classes | Antineoplastic Agent, Immune Suppressant |
| Drug Label | Zortress (everolimus) is a macrolide immunosuppressant.The chemical name of everolimus is(1R, 9S, 12S, 15R, 16E, 18R, 19R, 21R, 23S, 24E, 26E, 28E, 30S, 32S, 35R)-1, 18-dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-m... |
| Active Ingredient | Everolimus |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.5mg; 0.25mg; 0.75mg |
| Market Status | Prescription |
| Company | Novartis |
| 3 of 4 | |
|---|---|
| Drug Name | Afinitor |
| PubMed Health | Everolimus (By mouth) |
| Drug Classes | Antineoplastic Agent, Immune Suppressant |
| Drug Label | AFINITOR (everolimus), an inhibitor of mammalian target of rapamycin (mTOR), is an antineoplastic agent.The chemical name of everolimus is (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18- dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxy... |
| Active Ingredient | Everolimus |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 5mg; 7.5mg; 10mg |
| Market Status | Prescription |
| Company | Novartis |
| 4 of 4 | |
|---|---|
| Drug Name | Zortress |
| PubMed Health | Everolimus (By mouth) |
| Drug Classes | Antineoplastic Agent, Immune Suppressant |
| Drug Label | Zortress (everolimus) is a macrolide immunosuppressant.The chemical name of everolimus is(1R, 9S, 12S, 15R, 16E, 18R, 19R, 21R, 23S, 24E, 26E, 28E, 30S, 32S, 35R)-1, 18-dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-m... |
| Active Ingredient | Everolimus |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.5mg; 0.25mg; 0.75mg |
| Market Status | Prescription |
| Company | Novartis |
Immunosuppressive Agents
National Library of Medicine's Medical Subject Headings. Everolimus. Online file (MeSH, 2015). Available from, as of May 1, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Everolimus is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 18, 2015: https://clinicaltrials.gov/search/intervention=Everolimus
Afinitor is indicated for the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane, after failure of treatment with letrozole or anastrozole. /Included in US product label/
NIH; DailyMed. Current Medication Information for Afinitor (Everolimus) Tablet; Afinitor Disperz (Everolimus) Tablet, For Suspension (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2150f73a-179b-4afc-b8ce-67c85cc72f04
Afinitor Tablets and Afinitor Disperz are indicated in pediatric and adult patients with tuberous sclerosis complex (TSC) for the treatment of subependymal giant cell astrocytoma (SEGA) that requires therapeutic intervention but cannot be curatively resected. /Included in US product label/c
NIH; DailyMed. Current Medication Information for Afinitor (Everolimus) Tablet; Afinitor Disperz (Everolimus) Tablet, For Suspension (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2150f73a-179b-4afc-b8ce-67c85cc72f04
For more Therapeutic Uses (Complete) data for EVEROLIMUS (9 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: MALIGNANCIES AND SERIOUS INFECTIONS. Only physicians experienced in immunosuppressive therapy and management of transplant patients should prescribe Zortress. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient. Increased susceptibility to infection and the possible development of malignancies such as lymphoma and skin cancer may result from immunosuppression.
NIH; DailyMed. Current Medication Information for Zortress (Everolimus) Tablet (Updated: January 2015). Available from, as of May 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e082a024-7850-400b-a5c2-2a140612562a
/BOXED WARNING/ WARNING: KIDNEY GRAFT THROMBOSIS. An increased risk of kidney arterial and venous thrombosis, resulting in graft loss, was reported, mostly within the first 30 days post-transplantation.
NIH; DailyMed. Current Medication Information for Zortress (Everolimus) Tablet (Updated: January 2015). Available from, as of May 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e082a024-7850-400b-a5c2-2a140612562a
/BOXED WARNING/ WARNING: NEPHROTOXICITY. Increased nephrotoxicity can occur with use of standard doses of cyclosporine in combination with Zortress. Therefore reduced doses of cyclosporine should be used in combination with Zortress in order to reduce renal dysfunction. It is important to monitor the cyclosporine and everolimus whole blood trough concentrations.
NIH; DailyMed. Current Medication Information for Zortress (Everolimus) Tablet (Updated: January 2015). Available from, as of May 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e082a024-7850-400b-a5c2-2a140612562a
/BOXED WARNING/ WARNING: MORTALITY IN HEART TRANSPLANTATION. Increased mortality, often associated with serious infections, within the first three months post-transplantation was observed in a clinical trial of de novo heart transplant patients receiving immunosuppressive regimens with or without induction therapy. Use in heart transplantation is not recommended.
NIH; DailyMed. Current Medication Information for Zortress (Everolimus) Tablet (Updated: January 2015). Available from, as of May 19, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e082a024-7850-400b-a5c2-2a140612562a
For more Drug Warnings (Complete) data for EVEROLIMUS (32 total), please visit the HSDB record page.
Everolimus is indicated for the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane, after failure of treatment with letrozole or anastrozole. Indicated for the treatment of adult patients with progressive neuroendocrine tumors of pancreatic origin (PNET) with unresectable, locally advanced or metastatic disease. Indicated for the treatment of adult patients with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib. Indicated for the treatment of adult patients with renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery. Indicated in pediatric and adult patients with tuberous sclerosis complex (TSC) for the treatment of subependymal giant cell astrocytoma (SEGA) that requires therapeutic intervention but cannot be curatively resected.
FDA Label
* Hormone-receptor-positive advanced breast:
* cancer :
Afinitor is indicated for the treatment of hormone-receptor-positive, HER2/neu-negative advanced breast cancer , in combination with exemestane, in post-menopausal women without symptomatic visceral disease after recurrence or progression following a non-steroidal aromatase inhibitor.
* Neuroendocrine tumours of pancreatic origin:
Afinitor is indicated for the treatment of unresectable or metastatic, well or moderately differentiated neuroendocrine tumours of pancreatic origin in adults with progressive disease.
* Neuroendocrine tumours of gastrointestinal or lung origin:
Afinitor is indicated for the treatment of unresectable or metastatic, well-differentiated (Grade 1 or Grade 2) non-functional neuroendocrine tumours of gastrointestinal or lung origin in adults with progressive disease.
* Renal-cell carcinoma:
Afinitor is indicated for the treatment of patients with advanced renal-cell carcinoma, whose disease has progressed on or after treatment with VEGF-targeted therapy.
* Renal angiomyolipoma associated with tuberous sclerosis complex (TSC):
Votubia is indicated for the treatment of adult patients with renal angiomyolipoma associated with tuberous sclerosis complex (TSC) who are at risk of complications (based on factors such as tumour size or presence of aneurysm, or presence of multiple or bilateral tumours) but who do not require immediate surgery.
The evidence is based on analysis of change in sum of angiomyolipoma volume.
* Subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC):
Votubia is indicated for the treatment of patients with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not amenable to surgery.
The evidence is based on analysis of change in SEGA volume. Further clinical benefit, such as improvement in diseaserelated symptoms, has not been demonstrated.
Treatment of tuberous sclerosis complex
Treatment of thoracic neuroendocrine tumour
Carcinoid tumours
Prevention of rejection of transplanted liver, Prevention of rejection of transplanted heart, Prevention of rejection of transplanted kidney
Treatment of angiomyolipoma, Treatment of subependymal giant-cell astrocytoma
Renal cell carcinoma and pancreatic neuroendocrine tumour
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
MTOR Inhibitors
Agents that inhibit the activity of TOR SERINE-THREONINE KINASES. (See all compounds classified as MTOR Inhibitors.)
L01XE10
L01XE10
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EG - Mammalian target of rapamycin (mtor) kinase inhibitors
L01EG02 - Everolimus
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA18 - Everolimus
Absorption
In patients with advanced solid tumors, peak everolimus concentrations are reached 1 to 2 hours after administration of oral doses ranging from 5 mg to 70 mg. Following single doses, Cmax is dose-proportional between 5 mg and 10 mg. At doses of 20 mg and higher, the increase in Cmax is less than dose-proportional, however AUC shows dose-proportionality over the 5 mg to 70 mg dose range. Steady-state was achieved within 2 weeks following once-daily dosing. Dose Proportionality in Patients with SEGA (subependymal giant-cell astrocytomas) and TSC (tuberous sclerosis complex): In patients with SEGA and TSC, everolimus Cmin was approximately dose-proportional within the dose range from 1.35 mg/m2 to 14.4 mg/m2.
Route of Elimination
After a single dose of radiolabeled everolimus was given to transplant patients receiving cyclosporine, the majority (80%) of radioactivity was recovered from the feces and only a minor amount (5%) was excreted in urine.
Volume of Distribution
The blood-to-plasma ratio of everolimus is 17% to 73%.
Clearance
Following a 3 mg radiolabeled dose of everolimus, 80% of the radioactivity was recovered from the feces, while 5% was excreted in the urine.
The blood-to-plasma ratio of everolimus is concentration dependent ranging from 17% to 73% over the range of 5 ng/mL to 5000 ng/mL. Plasma protein binding is approximately 74% in healthy subjects and in patients with moderate hepatic impairment. The apparent distribution volume associated with the terminal phase (Vz/F) from a single-dose pharmacokinetic study in maintenance kidney transplant patients is 342 to 107 L (range 128 to 589 L).
NIH; DailyMed. Current Medication Information for Zortress (Everolimus) Tablet (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e082a024-7850-400b-a5c2-2a140612562a
The blood-to-plasma ratio of everolimus, which is concentration-dependent over the range of 5 to 5000 ng/mL, is 17% to 73%. The amount of everolimus confined to the plasma is approximately 20% at blood concentrations observed in cancer patients given Afinitor 10 mg/day. Plasma protein binding is approximately 74% both in healthy subjects and in patients with moderate hepatic impairment.
NIH; DailyMed. Current Medication Information for Afinitor (Everolimus) Tablet; Afinitor Disperz (Everolimus) Tablet, For Suspension (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2150f73a-179b-4afc-b8ce-67c85cc72f04
After administration of Afinitor tablets in patients with advanced solid tumors, peak everolimus concentrations are reached 1 to 2 hours after administration of oral doses ranging from 5 mg to 70 mg. Following single doses, Cmax is dose-proportional with daily dosing between 5 mg and 10 mg. With single doses of 20 mg and higher, the increase in Cmax is less than dose-proportional, however AUC shows dose-proportionality over the 5 mg to 70 mg dose range. Steady-state was achieved within 2 weeks following once-daily dosing.
NIH; DailyMed. Current Medication Information for Afinitor (Everolimus) Tablet; Afinitor Disperz (Everolimus) Tablet, For Suspension (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2150f73a-179b-4afc-b8ce-67c85cc72f04
No specific elimination studies have been undertaken in cancer patients. Following the administration of a 3 mg single dose of radiolabeled everolimus in patients who were receiving cyclosporine, 80% of the radioactivity was recovered from the feces, while 5% was excreted in the urine. The parent substance was not detected in urine or feces. The mean elimination half-life of everolimus is approximately 30 hours.
NIH; DailyMed. Current Medication Information for Afinitor (Everolimus) Tablet; Afinitor Disperz (Everolimus) Tablet, For Suspension (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2150f73a-179b-4afc-b8ce-67c85cc72f04
For more Absorption, Distribution and Excretion (Complete) data for EVEROLIMUS (7 total), please visit the HSDB record page.
Everolimus is a substrate of CYP3A4 and PgP (phosphoglycolate phosphatase). Three monohydroxylated metabolites, two hydrolytic ring-opened products, and a phosphatidylcholine conjugate of everolimus were the 6 primary metabolites detected in human blood. In vitro, everolimus competitively inhibited the metabolism of CYP3A4 and was a mixed inhibitor of the CYP2D6 substrate dextromethorphan.
Everolimus is a substrate of CYP3A4 and PgP. Following oral administration, everolimus is the main circulating component in human blood. Six main metabolites of everolimus have been detected in human blood, including three monohydroxylated metabolites, two hydrolytic ring-opened products, and a phosphatidylcholine conjugate of everolimus. These metabolites were also identified in animal species used in toxicity studies, and showed approximately 100-times less activity than everolimus itself.
NIH; DailyMed. Current Medication Information for Afinitor (Everolimus) Tablet; Afinitor Disperz (Everolimus) Tablet, For Suspension (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2150f73a-179b-4afc-b8ce-67c85cc72f04
Everolimus has known human metabolites that include (1R,9S,12S,15R,16Z,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-Dihydroxy-12-[(2R)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone and (1R,9S,12S,15R,16Z,18R,19R,21R,23S,24E,30S,32S,35R)-1,18-dihydroxy-12-[(2R)-1-[(1S,3R,4R)-3-hydroxy-4-(2-hydroxyethoxy)cyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
~30 hours.
The mean elimination half-life of everolimus is approximately 30 hours.
NIH; DailyMed. Current Medication Information for Afinitor (Everolimus) Tablet; Afinitor Disperz (Everolimus) Tablet, For Suspension (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2150f73a-179b-4afc-b8ce-67c85cc72f04
Everolimus is a mTOR inhibitor that binds with high affinity to the FK506 binding protein-12 (FKBP-12), thereby forming a drug complex that inhibits the activation of mTOR. This inhibition reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest and apoptosis. Everolimus also inhibits the expression of hypoxia-inducible factor, leading to a decrease in the expression of vascular endothelial growth factor. The result of everolimus inhibition of mTOR is a reduction in cell proliferation, angiogenesis, and glucose uptake.
Mechanistic target of rapamycin (mTOR) is a serine-threonine kinase that functions via two multiprotein complexes, namely mTORC1 and mTORC2, each characterized by different binding partners that confer separate functions. mTORC1 function is tightly regulated by PI3-K/Akt and is sensitive to rapamycin. mTORC2 is sensitive to growth factors, not nutrients, and is associated with rapamycin-insensitivity. mTORC1 regulates protein synthesis and cell growth through downstream molecules: 4E-BP1 (also called EIF4E-BP1) and S6K. Also, mTORC2 is thought to modulate growth factor signaling by phosphorylating the C-terminal hydrophobic motif of some AGC kinases such as Akt and SGK. Recent evidence has suggested that mTORC2 may play an important role in maintenance of normal as well as cancer cells by virtue of its association with ribosomes, which may be involved in metabolic regulation of the cell. Rapamycin (sirolimus) and its analogs known as rapalogues, such as RAD001 (everolimus) and CCI-779 (temsirolimus), suppress mTOR activity through an allosteric mechanism that acts at a distance from the ATP-catalytic binding site, and are considered incomplete inhibitors. Moreover, these compounds suppress mTORC1-mediated S6K activation, thereby blocking a negative feedback loop, leading to activation of mitogenic pathways promoting cell survival and growth. Consequently, mTOR is a suitable target of therapy in cancer treatments. However, neither of these complexes is fully inhibited by the allosteric inhibitor rapamycin or its analogs. In recent years, new pharmacologic agents have been developed which can inhibit these complexes via ATP-binding mechanism, or dual inhibition of the canonical PI3-K/Akt/mTOR signaling pathway. These compounds include WYE-354, KU-003679, PI-103, Torin1, and Torin2, which can target both complexes or serve as a dual inhibitor for PI3-K/mTOR. This investigation describes the mechanism of action of pharmacological agents that effectively target mTORC1 and mTORC2 resulting in suppression of growth, proliferation, and migration of tumor and cancer stem cells.
PMID:25442674 Jhanwar-Uniyal M et al; Adv Biol Regul 57: 64-74 (2015)
Mammalian target of rapamycin (mTOR) inhibitors have anti-tumor effects against renal cell carcinoma, pancreatic neuroendocrine cancer and breast cancer. In this study, we analyzed the antitumor effects of mTOR inhibitors in small cell lung cancer (SCLC) cells and sought to clarify the mechanism of resistance to mTOR inhibitors. We analyzed the antitumor effects of three mTOR inhibitors including everolimus in 7 SCLC cell lines by MTS assay. Gene-chip analysis, receptor tyrosine kinases (RTK) array and Western blotting analysis were performed to identify molecules associated with resistance to everolimus. Only SBC5 cells showed sensitivity to everolimus by MTS assay. We established two everolimus resistant-SBC5 cell lines (SBC5 R1 and SBC5 R10) by continuous exposure to increasing concentrations of everolimus stepwise. SPP1 and MYC were overexpressed in both SBC5 R1 and SBC5 R10 by gene-chip analysis. High expression levels of eukaryotic translation initiation factor 4E (eIF4E) were observed in 5 everolimus-resistant SCLC cells and SBC5 R10 cells by Western blotting. MYC siRNA reduced eIF4E phosphorylation in SBC5 cells, suggesting that MYC directly activates eIF4E by an mTOR-independent bypass pathway. Importantly, after reduction of MYC or eIF4E by siRNAs, the SBC5 parent and two SBC5-resistant cells displayed increased sensitivity to everolimus relative to the siRNA controls. These findings suggest that eIF4E has been shown to be an important factor in the resistance to everolimus in SCLC cells. Furthermore, a link between MYC and mTOR-independent eIF4E contribute to the resistance to everolimus in SCLC cells. Control of the MYC-eIF4E axis may be a novel therapeutic strategy for everolimus action in SCLC.
PMID:25884680 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4414307 Matsumoto M et al; BMC Cancer. 2015 Apr 9;15:241. doi: 10.1186/s12885-015-1202-4
Everolimus inhibits antigenic and interleukin (IL-2 and IL-15) stimulated activation and proliferation of T and B lymphocytes. In cells, everolimus binds to a cytoplasmic protein, the FK506 Binding Protein-12 (FKBP-12), to form an immunosuppressive complex (everolimus: FKBP-12) that binds to and inhibits the mammalian Target Of Rapamycin (mTOR), a key regulatory kinase. In the presence of everolimus phosphorylation of p70 S6 ribosomal protein kinase (p70S6K), a substrate of mTOR, is inhibited. Consequently, phosphorylation of the ribosomal S6 protein and subsequent protein synthesis and cell proliferation are inhibited. The everolimus: FKBP-12 complex has no effect on calcineurin activity. In rats and nonhuman primate models, everolimus effectively reduces kidney allograft rejection resulting in prolonged graft survival.
NIH; DailyMed. Current Medication Information for Zortress (Everolimus) Tablet (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e082a024-7850-400b-a5c2-2a140612562a
Everolimus is an inhibitor of mammalian target of rapamycin (mTOR), a serine-threonine kinase, downstream of the PI3K/AKT pathway. The mTOR pathway is dysregulated in several human cancers. Everolimus binds to an intracellular protein, FKBP-12, resulting in an inhibitory complex formation with mTOR complex 1 (mTORC1) and thus inhibition of mTOR kinase activity. Everolimus reduced the activity of S6 ribosomal protein kinase (S6K1) and eukaryotic initiation factor 4E-binding protein (4E-BP1), downstream effectors of mTOR, involved in protein synthesis. S6K1 is a substrate of mTORC1 and phosphorylates the activation domain 1 of the estrogen receptor which results in ligand-independent activation of the receptor. In addition, everolimus inhibited the expression of hypoxia-inducible factor (e.g., HIF-1) and reduced the expression of vascular endothelial growth factor (VEGF). Inhibition of mTOR by everolimus has been shown to reduce cell proliferation, angiogenesis, and glucose uptake in in vitro and/or in vivo studies. Constitutive activation of the PI3K/Akt/mTOR pathway can contribute to endocrine resistance in breast cancer. In vitro studies show that estrogen-dependent and HER2+ breast cancer cells are sensitive to the inhibitory effects of everolimus, and that combination treatment with everolimus and Akt, HER2, or aromatase inhibitors enhances the anti-tumor activity of everolimus in a synergistic manner. Two regulators of mTORC1 signaling are the oncogene suppressors tuberin-sclerosis complexes 1 and 2 (TSC1, TSC2). Loss or inactivation of either TSC1 or TSC2 leads to activation of downstream signaling. In TSC, a genetic disorder, inactivating mutations in either the TSC1 or the TSC2 gene lead to hamartoma formation throughout the body.
NIH; DailyMed. Current Medication Information for Afinitor (Everolimus) Tablet; Afinitor Disperz (Everolimus) Tablet, For Suspension (Updated: January 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2150f73a-179b-4afc-b8ce-67c85cc72f04

235.2k
51 - 200
108.7
25.6M
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|---|---|---|
| CHILE | 31.71 | 2,55,674.0 | 11 - 50 |
| CZECH REPUBLIC | 0.91 | 2,46,809.9 | 11 - 50 |
| FRANCE | 14.88 | 2,62,788.8 | 11 - 50 |
| INDIA | 19.78 | 3,39,303.4 | <10 |
| RUSSIA | 9.84 | 1,15,500.0 | <10 |
| KOREA,REPUBLIC O | 0.97 | 4,81,680.5 | <10 |
| BRAZIL | 0.92 | 3,07,418.4 | <10 |
| CROATIA | 3.01 | 2,44,589.2 | <10 |
| EGYPT | 0.96 | 2,47,827.9 | <10 |
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
19
PharmaCompass offers a list of Everolimus API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Everolimus manufacturer or Everolimus supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Everolimus manufacturer or Everolimus supplier.
PharmaCompass also assists you with knowing the Everolimus API Price utilized in the formulation of products. Everolimus API Price is not always fixed or binding as the Everolimus Price is obtained through a variety of data sources. The Everolimus Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Everolimus manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Everolimus, including repackagers and relabelers. The FDA regulates Everolimus manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Everolimus API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Everolimus manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Everolimus supplier is an individual or a company that provides Everolimus active pharmaceutical ingredient (API) or Everolimus finished formulations upon request. The Everolimus suppliers may include Everolimus API manufacturers, exporters, distributors and traders.
click here to find a list of Everolimus suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Everolimus DMF (Drug Master File) is a document detailing the whole manufacturing process of Everolimus active pharmaceutical ingredient (API) in detail. Different forms of Everolimus DMFs exist exist since differing nations have different regulations, such as Everolimus USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Everolimus DMF submitted to regulatory agencies in the US is known as a USDMF. Everolimus USDMF includes data on Everolimus's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Everolimus USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Everolimus suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Everolimus Drug Master File in Japan (Everolimus JDMF) empowers Everolimus API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Everolimus JDMF during the approval evaluation for pharmaceutical products. At the time of Everolimus JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Everolimus suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Everolimus Drug Master File in Korea (Everolimus KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Everolimus. The MFDS reviews the Everolimus KDMF as part of the drug registration process and uses the information provided in the Everolimus KDMF to evaluate the safety and efficacy of the drug.
After submitting a Everolimus KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Everolimus API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Everolimus suppliers with KDMF on PharmaCompass.
A Everolimus CEP of the European Pharmacopoeia monograph is often referred to as a Everolimus Certificate of Suitability (COS). The purpose of a Everolimus CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Everolimus EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Everolimus to their clients by showing that a Everolimus CEP has been issued for it. The manufacturer submits a Everolimus CEP (COS) as part of the market authorization procedure, and it takes on the role of a Everolimus CEP holder for the record. Additionally, the data presented in the Everolimus CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Everolimus DMF.
A Everolimus CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Everolimus CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Everolimus suppliers with CEP (COS) on PharmaCompass.
A Everolimus written confirmation (Everolimus WC) is an official document issued by a regulatory agency to a Everolimus manufacturer, verifying that the manufacturing facility of a Everolimus active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Everolimus APIs or Everolimus finished pharmaceutical products to another nation, regulatory agencies frequently require a Everolimus WC (written confirmation) as part of the regulatory process.
click here to find a list of Everolimus suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Everolimus as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Everolimus API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Everolimus as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Everolimus and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Everolimus NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Everolimus suppliers with NDC on PharmaCompass.
Everolimus Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Everolimus GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Everolimus GMP manufacturer or Everolimus GMP API supplier for your needs.
A Everolimus CoA (Certificate of Analysis) is a formal document that attests to Everolimus's compliance with Everolimus specifications and serves as a tool for batch-level quality control.
Everolimus CoA mostly includes findings from lab analyses of a specific batch. For each Everolimus CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Everolimus may be tested according to a variety of international standards, such as European Pharmacopoeia (Everolimus EP), Everolimus JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Everolimus USP).
