
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Finished Drug Prices
NA
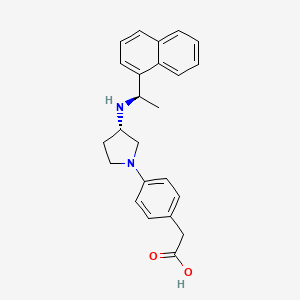

1. (4-((3s)-3-(((1r)-1-(1-naphthyl)ethyl)amino)-1-pyrrolidinyl)phenyl)acetic Acid
1. 870964-67-3
2. Evocalcet [inn]
3. Mt-4580
4. Khk-7580
5. Khk7580
6. E58mlh082p
7. 4-[(3s)-3-[[(1r)-1-(1-naphthalenyl)ethyl]amino]-1-pyrrolidinyl]benzeneaceticacid
8. 2-[4-[(3s)-3-[[(1r)-1-naphthalen-1-ylethyl]amino]pyrrolidin-1-yl]phenyl]acetic Acid
9. 4-[(3s)-3-[[(1r)-1-(1-naphthalenyl)ethyl]amino]-1-pyrrolidinyl]benzeneacetic Acid
10. Benzeneacetic Acid, 4-((3s)-3-(((1r)-1-(1-naphthalenyl)ethyl)amino)-1-pyrrolidinyl)-
11. 2-(4-((s)-3-(((r)-1-(naphthalen-1-yl)ethyl)amino)pyrrolidin-1-yl)phenyl)acetic Acid
12. 2-[4-[(3s)-3-[[(1r)-1-naphthalen-1-ylethyl]amino]pyrrolidin-1-yl]phenyl]ethanoic Acid
13. Unii-e58mlh082p
14. Evocalcet?
15. H43
16. Orkedia (tn)
17. Evocalcet (jan/inn)
18. Evocalcet [jan]
19. Evocalcet [mi]
20. Evocalcet [who-dd]
21. Gtpl9042
22. Chembl4297621
23. Schembl14668291
24. Khk7580khk7580
25. Dtxsid101132784
26. Vjb96467
27. Akos040741717
28. Db12388
29. Khk7580; Mt-4580
30. Hy-17613
31. Ms-26062
32. Cs-0014698
33. Ns00073438
34. D11063
35. G15959
36. Q27077250
37. (4-((3s)-3-(((1r)-1-(1-naphthyl)ethyl)amino)-1-pyrrolidinyl)phenyl)acetic Acid
| Molecular Weight | 374.5 g/mol |
|---|---|
| Molecular Formula | C24H26N2O2 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 52.6 |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 517 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
H - Systemic hormonal preparations, excl. sex hormones and insulins
H05 - Calcium homeostasis
H05B - Anti-parathyroid agents
H05BX - Other anti-parathyroid agents
H05BX06 - Evocalcet

Registration Number : 229MF10027
Registrant's Address : Osaka Prefecture, Osaka City, Chuo Ward, Doshomachi 3-2-10
Initial Date of Registration : 2017-02-08
Latest Date of Registration : --





 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Japan
Brand Name : Orkedia
Dosage Form : Tablet
Dosage Strength : 4MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Japan

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Japan
Brand Name : Orkedia
Dosage Form : Tablet
Dosage Strength : 4MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Japan

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 4MG
Brand Name : Orkedia
Approval Date :
Application Number :
Registration Country : Japan

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Main Therapeutic Indication : Hormonal Disorders
Currency : USD
2020 Revenue in Millions : 86
2019 Revenue in Millions : 65
Growth (%) : 32

Main Therapeutic Indication : Hormonal Disorders
Currency : USD
2021 Revenue in Millions : 84
2020 Revenue in Millions : 86
Growth (%) : 9

Main Therapeutic Indication : Endocrinology
Currency : USD
2022 Revenue in Millions : 78
2021 Revenue in Millions : 84
Growth (%) : -7

Main Therapeutic Indication : Endocrinology
Currency : USD
2023 Revenue in Millions : 71
2022 Revenue in Millions : 78
Growth (%) : 3

Main Therapeutic Indication : Hormonal Disorders
Currency : USD
2019 Revenue in Millions : 64
2018 Revenue in Millions : 22
Growth (%) : 188

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?