
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
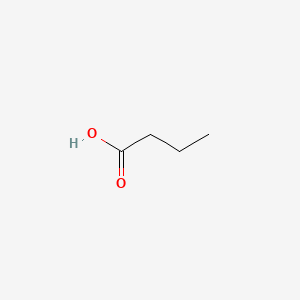

1. Acid, Butanoic
2. Acid, Butyric
3. Butanoic Acid
4. Butyrate, Magnesium
5. Butyrate, Sodium
6. Butyric Acid Magnesium Salt
7. Butyric Acid, Sodium Salt
8. Dibutyrate, Magnesium
9. Magnesium Butyrate
10. Magnesium Dibutyrate
11. Sodium Butyrate
1. Butanoic Acid
2. 107-92-6
3. N-butyric Acid
4. N-butanoic Acid
5. Propylformic Acid
6. Ethylacetic Acid
7. 1-propanecarboxylic Acid
8. Butanic Acid
9. Butyrate
10. 1-butyric Acid
11. Buttersaeure
12. Butanoate
13. Butyric Acid (natural)
14. Kyselina Maselna
15. Propanecarboxylic Acid
16. Buttersaeure [german]
17. 1-butanoic Acid
18. Fema No. 2221
19. Fema Number 2221
20. Kyselina Maselna [czech]
21. Ccris 6552
22. Hsdb 940
23. Butoic Acid
24. 2-butanoate
25. Nsc 8415
26. Mfcd00002814
27. Un2820
28. Ai3-15306
29. C4:0
30. Ch3-[ch2]2-cooh
31. 40uir9q29h
32. 67254-79-9
33. Chebi:30772
34. Nsc8415
35. Butanate
36. Propylformate
37. Nsc-8415
38. Butyrate Sodium
39. 1-butanoate
40. Propanecarboxylate
41. 1-butyrate
42. Butyric Acid [un2820] [corrosive]
43. Sodium N-butyrate
44. 1-propanecarboxylate
45. Dsstox_cid_1515
46. Dsstox_rid_76192
47. Dsstox_gsid_21515
48. Bua
49. Acide Butyrique
50. Fatty Acids
51. Cas-107-92-6
52. Butyric Acid (normal)
53. Einecs 203-532-3
54. Brn 0906770
55. Unii-40uir9q29h
56. Sodium-butyrate
57. Acide Butanoique
58. Honey Robber
59. Butyricum Acidum
60. Ethyl Acetic Acid
61. 1ugp
62. 3umq
63. Butanoic Acid, 4
64. Nat. Butyric Acid
65. Fatty Acid,vegetable
66. Tnfa + Nabut
67. Butyrate, Sodium Salt
68. Butyric Acid [un2820] [corrosive]
69. Butyric_acid
70. N-c3h7cooh
71. Tnfa + Sodium Butyrate
72. Butyric Acid, >=99%
73. Normal Butyric Acid
74. Bmse000402
75. Butyric Acid [mi]
76. Ec 203-532-3
77. Natural Butyric Acid
78. Ncimech_000707
79. Butyric Acid [fcc]
80. Wln: Qv3
81. Butyric Acid [hsdb]
82. Butyric Acid [inci]
83. Butyric Acid [vandf]
84. 4-02-00-00779 (beilstein Handbook Reference)
85. Chembl14227
86. Butyric Acid [who-dd]
87. Butyric Acid, >=99%, Fg
88. N-butyric Acid [fhfi]
89. Gtpl1059
90. Butyricum Acidum [hpus]
91. Dtxsid8021515
92. Bdbm26109
93. Butyric Acid, Analytical Standard
94. Bio1_000444
95. Bio1_000933
96. Bio1_001422
97. N-butyric Acid, Ethyl Acetic Acid
98. Zinc895132
99. Str06290
100. Tox21_202382
101. Tox21_300164
102. Ccg-35836
103. Lmfa01010004
104. Stl169349
105. Akos000118961
106. Db03568
107. Un 2820
108. Ncgc00247914-01
109. Ncgc00247914-02
110. Ncgc00247914-05
111. Ncgc00253919-01
112. Ncgc00259931-01
113. Bp-21420
114. Nci60_001424
115. Butyric Acid, Natural, >=99%, Fcc, Fg
116. B0754
117. Ft-0623295
118. Ft-0686717
119. Butyric Acid 1000 Microg/ml In Acetonitrile
120. Butyric Acid, Saj Special Grade, >=99.5%
121. C00246
122. Q193213
123. W-108732
124. Brd-k05878375-236-02-4
125. 9b27b3d0-9643-40ec-9a5f-7ca1a6ed7f9f
126. F2191-0094
127. Z955123634
128. Butanoic Acid, Butanic Acid, N-butyric Acid, Ethylacetic Acid, Propylformic Acid, 1-propanecarboxylic Acid
| Molecular Weight | 88.11 g/mol |
|---|---|
| Molecular Formula | C4H8O2 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 88.052429494 g/mol |
| Monoisotopic Mass | 88.052429494 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 49.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Mesh Heading: histamine antagonists
National Library of Medicine, SIS; ChemIDplus Record for Butyric Acid (107-92-6). Available from, as of April 13, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Histamine Antagonists
Drugs that bind to but do not activate histamine receptors, thereby blocking the actions of histamine or histamine agonists. Classical antihistaminics block the histamine H1 receptors only. (See all compounds classified as Histamine Antagonists.)
Butyric acid is readily absorbed from the gastrointestinal tract ...
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:709
A pharmacokinetics study was performed by injecting butyric acid as sodium or arginine salts for possible antitumor therapies. In the case of 1-(14)C-labelled butyrate, the appearance of radioactivity in the blood of injected mice is rapid and some of it is maintained for relatively long periods in different organs, mainly the liver. However, no precision can be given about the structure of radioactive compounds in blood and tissues. Using GLC, the metabolism of butyrate in both animals and man were studied. In mice and rabbits, the half-life is less than 5 min. In man, the butyric acid elimination curve can be divided into two parts corresponding to two half-lives: for the first (0.5 min), the slope suggests an accelerated excretion, while for the following (13.7 min), a slow plateau is observed. The rapid elimination of butyrate is a limiting factor for practical applications. However, the lack of toxicity supports its use in human therapy.
PMID:2667816 Daniel P et al; Clin Chim Acta 181 (3): 255-63 (1989)
Butyric acid is ... rapidly metabolized by the liver. In rats a considerable portion ... is metabolized to acetic acid. Butyric acid metabolism gives rise to ketone bodies (beta-hydroxybutyrate, acetoacetate, acetone) and acetic acid, which may be excreted in the urine or incorporated into normal processes of fat metabolism.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:709
The metabolism of carboxyl-labeled butyric acid by liver tissue was investigated in vitro. It was shown that the test substance was converted to ketone bodies mainly by fission into 2-carbon chains with subsequent recombination, and to a lesser extent by direct beta-oxidation.
European Chemicals Bureau; IUCLID Dataset, Butyric acid (107-92-6) (2000 CD-ROM edition). Available from, as of April 20, 2006: https://esis.jrc.ec.europa.eu/
In isolated animal tissues butyric acid was oxidized to acetoacetic and beta-hydroxybutyric acid. The formation of carbohydrate and complete oxidation were also reported. Besides the formation of beta-hydroxybutyric acid the formation of ketone bodies represents possibly an alternative path after oxidation at the beta-carbon atom of butyric acid.
European Chemicals Bureau; IUCLID Dataset, Butyric acid (107-92-6) (2000 CD-ROM edition). Available from, as of April 20, 2006: https://esis.jrc.ec.europa.eu/
... Following butyraldehyde intake ... it is oxidized by aldehyde dehydrogenase, largely in the liver but also in other tissues. Butyric acid undergoes further oxidation via the Krebs cycle, or it may be conjugated with glutathione.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:978
Diet, especially the amount of starch and dietary fiber which escape digestion in the small intestine are major determinants of colon function in man. These carbohydrates are the principal substrates for fermentation by the large bowel flora. Carbohydrate fermentation results in lowered caecal pH and the production of short chain fatty acids of which butyric acid may protect the colon epithelium from dysplastic change. Protein digestion and amino acid fermentation also occur in the large bowel but the nature of its endproducts varies in relation to the amount of carbohydrate available. During active carbohydrate breakdown amino acid fermentation endproducts such as ammonia are used by the bacteria for protein synthesis during microbial growth, but in carbon limited fermentation amines, ammonia, phenols and indoles, etc, accumulate. Fermentation also results in changes in colon pH which alters the metabolism of bile acids, nitrate, sulfate and other substances. Fermentation is thus controlled to a great extent by substrate availability, especially of carbohydrates which are derived from the diet. The potential to induce mutagenic change in colon epithelial cells and promote tumor growth may readily be influenced by diet.
PMID:2838168 Cummings JH, Bingham SA; Cancer Surv 6 (4): 601-21 (1987)
Butyric acid has two contrasting functional roles. As a product of fermentation within the human colon, it serves as the most important energy source for normal colorectal epithelium. It also promotes the differentiation of cultured malignant cells. A switch from aerobic to anaerobic metabolism accompanies neoplastic transformation in the colorectum. The separate functional roles for n-butyrate may reflect the different metabolic activities of normal and neoplastic tissues. Deficiency of n-butyrate, coupled to the increased energy requirements of neoplastic tissue, may promote the switch to anaerobic metabolism.
PMID:3916695 Jass JR; Med Hypotheses 18 (2): 113-8 (1985)
Treatment of cultured HeLa cells with 5 mM butyrate caused an inhibition of growth as well as extensive chemical and morphological differentiation. Lysosomal enzyme activity changes are associated with both normal and neoplastic growth as well as many aspects of the neoplastic process. The comparative ultrastructural results showed that the butyrate treated cells had a more extensive internal membranous system than the untreated cells, whereas other organelles seemed unaffected. The histochemical localization of lysosomal acid phosphatase showed a 2-fold increase in particulate reaction product in the butyrate-treated HeLa cells. Butyrate treatment may prevent sublethal autolysis by arresting the leakage of the lysosomal enzymes from the lysosome into the cytosol and thus allowing the cell to differentiate chemically and morphologically.
Kelly RE; In Vitro 21 (7): 373-81 (1985)
Exposure of the rat glioma C6 cell line to butyric acid increased levels of L-triiodothyronine in the nuclear and extranuclear compartments. The increase in nuclear binding was not merely a reflection of the higher cellular hormone content, and Scatchard analysis of L-triiodothyronine binding to isolated nuclei revealed that butyric acid increased receptor number without changing affinity. The effect on the receptor was quantitatively important: a 48 hr incubation with 2 mM butyric acid increased nuclear binding by 2-3 fold, and 5 mM butyrate by 35 fold. Butyric acid increased receptor levels by decreasing receptor degradation, since the apparent half-life of receptor disappearance increased by approximately 3 fold in cells incubated with 2 mM butyric acid for 48 hr. Butyric acid had little effect in increasing the level of multiacetylated forms of H3 and H4 histone when studied in acid-urea gels, but it markedly inhibited the turnover of (3)H-acetate from the histone fraction. There was a striking similarity in the dose-response of butyric acid for increasing receptor levels and inhibiting histone deacetylation. Furthermore, a very close correlation between receptor levels and (3)H-acetate release was also found when different short-chain fatty acids were used.
PMID:3771518 Ortiz CJ et al; J Biol Chem 261 (30): 13997-4004 (1986)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
28
PharmaCompass offers a list of Sodium Butyrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Butyrate manufacturer or Sodium Butyrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Butyrate manufacturer or Sodium Butyrate supplier.
PharmaCompass also assists you with knowing the Sodium Butyrate API Price utilized in the formulation of products. Sodium Butyrate API Price is not always fixed or binding as the Sodium Butyrate Price is obtained through a variety of data sources. The Sodium Butyrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fatty Acids manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fatty Acids, including repackagers and relabelers. The FDA regulates Fatty Acids manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fatty Acids API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fatty Acids manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fatty Acids supplier is an individual or a company that provides Fatty Acids active pharmaceutical ingredient (API) or Fatty Acids finished formulations upon request. The Fatty Acids suppliers may include Fatty Acids API manufacturers, exporters, distributors and traders.
click here to find a list of Fatty Acids suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fatty Acids DMF (Drug Master File) is a document detailing the whole manufacturing process of Fatty Acids active pharmaceutical ingredient (API) in detail. Different forms of Fatty Acids DMFs exist exist since differing nations have different regulations, such as Fatty Acids USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fatty Acids DMF submitted to regulatory agencies in the US is known as a USDMF. Fatty Acids USDMF includes data on Fatty Acids's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fatty Acids USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fatty Acids suppliers with USDMF on PharmaCompass.
A Fatty Acids CEP of the European Pharmacopoeia monograph is often referred to as a Fatty Acids Certificate of Suitability (COS). The purpose of a Fatty Acids CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Fatty Acids EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Fatty Acids to their clients by showing that a Fatty Acids CEP has been issued for it. The manufacturer submits a Fatty Acids CEP (COS) as part of the market authorization procedure, and it takes on the role of a Fatty Acids CEP holder for the record. Additionally, the data presented in the Fatty Acids CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Fatty Acids DMF.
A Fatty Acids CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Fatty Acids CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Fatty Acids suppliers with CEP (COS) on PharmaCompass.
Fatty Acids Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fatty Acids GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fatty Acids GMP manufacturer or Fatty Acids GMP API supplier for your needs.
A Fatty Acids CoA (Certificate of Analysis) is a formal document that attests to Fatty Acids's compliance with Fatty Acids specifications and serves as a tool for batch-level quality control.
Fatty Acids CoA mostly includes findings from lab analyses of a specific batch. For each Fatty Acids CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fatty Acids may be tested according to a variety of international standards, such as European Pharmacopoeia (Fatty Acids EP), Fatty Acids JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fatty Acids USP).
