
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
South Africa

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
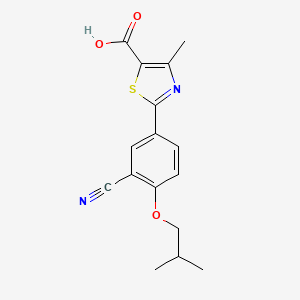

1. 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylic Acid
2. 6720, Tei
3. Tei 6720
4. Tei-6720
5. Tei6720
6. Uloric
1. 144060-53-7
2. Adenuric
3. Uloric
4. 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylic Acid
5. Tei 6720
6. Feburic
7. Tei-6720
8. Tmx 67
9. Tmx-67
10. 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-1,3-thiazole-5-carboxylic Acid
11. Zurig
12. C16h16n2o3s
13. Febuxostat (uloric)
14. Nsc-758874
15. 2-(3-cyano-4-isobutoxy-phenyl)-4-methyl-5-thiazole-carboxylic Acid
16. Chembl1164729
17. 101v0r1n2e
18. 5-thiazolecarboxylic Acid, 2-(3-cyano-4-(2-methylpropoxy)phenyl)-4-methyl-
19. Febuxostat [usan]
20. Ncgc00182059-02
21. Dsstox_cid_28576
22. Dsstox_rid_82848
23. Dsstox_gsid_48650
24. 5-thiazolecarboxylic Acid, 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-
25. Smr002529566
26. Uloric (tn)
27. Cas-144060-53-7
28. 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazolecarboxylic Acid
29. 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylic Acid
30. Donifoxate
31. Febuxostatum
32. Febuday
33. Goturic
34. Febric
35. Goutex
36. Febuxostat (jan/usan/inn)
37. Febuxostat [usan:inn:ban]
38. Unii-101v0r1n2e
39. Febuxostat- Bio-x
40. Feburic (tn)
41. Spiramycinadipate
42. 111ge013
43. Febuxostat [mi]
44. Febuxostat [inn]
45. Febuxostat [jan]
46. S1547
47. Febuxostat [vandf]
48. 2-(3-cyano-4-(2-methylpropoxy)phenyl)-4-methylthiazole-5-carboxylic Acid
49. Febuxostat [mart.]
50. Febuxostat [who-dd]
51. Febuxostat,uloric, Tmx-67
52. Mls004774136
53. Mls006011568
54. Febuxostat [ema Epar]
55. Schembl249339
56. Gtpl6817
57. Zinc5423
58. Thyl-thiazole-5-carboxylic Acid
59. Dtxsid8048650
60. Febuxostat [orange Book]
61. Febuxostat, >=98% (hplc)
62. Chebi:31596
63. Bcpp000233
64. Hms3264c20
65. Hms3655c03
66. Hms3673m21
67. Hms3743i09
68. Hms3868j03
69. Mx-67
70. Pharmakon1600-01504286
71. Act06289
72. Bcp02342
73. Wzb81950
74. Tox21_113004
75. Ac-425
76. Bbl036503
77. Bdbm50320491
78. Fd7322
79. Mfcd00871598
80. Nsc758874
81. Stl559020
82. Akos015841695
83. Tox21_113004_1
84. Bcp9000679
85. Bs-1018
86. Ccg-213303
87. Cs-0403
88. Db04854
89. Nsc 758874
90. Pb33929
91. 2-(3-cyano-4-isobutoxy-phenyl)-4-me
92. Ncgc00182059-03
93. Bc164443
94. Hy-14268
95. Am20090760
96. F0847
97. Ft-0601639
98. Sw219283-1
99. D01206
100. Ab01274796-01
101. Ab01274796_02
102. Ab01274796_03
103. 060f537
104. Q417296
105. Sr-01000940023
106. Q-100164
107. Sr-01000940023-2
108. Brd-k48367671-001-01-8
109. Z1550648761
110. 2-(3-cyano-4-isobutyloxy)-phenyl-4-methyl-5-thiazolecarboxylic Acid
111. 2-(3-cyano-4-isobutoxyphenyl)-4-methyl- 1,3-thiazole-5-carboxylic Acid
112. 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-1,3-thiazole-5-carboxylic Acid
| Molecular Weight | 316.4 g/mol |
|---|---|
| Molecular Formula | C16H16N2O3S |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 316.08816355 g/mol |
| Monoisotopic Mass | 316.08816355 g/mol |
| Topological Polar Surface Area | 111 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 448 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Uloric |
| PubMed Health | Febuxostat (By mouth) |
| Drug Classes | Antigout |
| Drug Label | ULORIC (febuxostat) is a xanthine oxidase inhibitor. The active ingredient in ULORIC is 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid, with a molecular weight of 316.38. The empirical formula is C16H16N2O3S.The chemical st... |
| Active Ingredient | Febuxostat |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 80mg; 40mg |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Uloric |
| PubMed Health | Febuxostat (By mouth) |
| Drug Classes | Antigout |
| Drug Label | ULORIC (febuxostat) is a xanthine oxidase inhibitor. The active ingredient in ULORIC is 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid, with a molecular weight of 316.38. The empirical formula is C16H16N2O3S.The chemical st... |
| Active Ingredient | Febuxostat |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 80mg; 40mg |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
Febuxostat is indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated dose of [allopurinol], who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable. It is not recommended for the treatment of asymptomatic hyperuricemia or secondary hyperuricemia.
FDA Label
Febuxostat Mylan is indicated for the prevention and treatment of hyperuricaemia in adult patients undergoing chemotherapy for haematologic malignancies at intermediate to high risk of Tumor Lysis Syndrome (TLS).
Febuxostat Mylan is indicated for the treatment of chronic hyperuricaemia in conditions where urate deposition has already occurred (including a history, or presence of, tophus and/or gouty arthritis ).
Febuxostat Mylan is indicated in adults.
Febuxostat Krka is indicated for the treatment of chronic hyperuricaemia in conditions where urate deposition has already occurred (including a history, or presence of, tophus and/or gouty arthritis ).
Febuxostat Krka is indicated in adults.
80 mg strength:
- Treatment of chronic hyperuricaemia in conditions where urate deposition has already occurred (including a history, or presence of, tophus and/or gouty arthritis ).
- Adenuric is indicated in adults.
120 mg strength:
- Adenuric is indicated for the treatment of chronic hyperuricaemia in conditions where urate deposition has already occurred (including a history, or presence of, tophus and/or gouty arthritis ).
- Adenuric is indicated for the prevention and treatment of hyperuricaemia in adult patients undergoing chemotherapy for haematologic malignancies at intermediate to high risk of Tumor Lysis Syndrome (TLS).
- Adenuric is indicated in adults.
Prevention of hyperuricaemia
Febuxostat is a novel, selective xanthine oxidase/dehydrogenase inhibitor that works by decreasing serum uric acid in a dose-dependent manner. In healthy subjects, febuxostat decreased the mean serum uric acid and serum xanthine concentrations, as well as the total urinary uric acid excretion. Febuxostat at daily doses of 40-80 mg reduced the 24-hour mean serum uric acid concentrations by 40 to 55%. Closely related to the drug-induced reduction of serum uric acid levels and mobilization of urate crystals in tissue deposits, febuxostat is associated with gout flares. Unlike [allopurinol] and [oxypurinol], febuxostat has no inhibitory actions against other enzymes involved in purine and pyrimidine synthesis and metabolism, because it does not structurally resemble purines or pyrimidines.
Gout Suppressants
Agents that increase uric acid excretion by the kidney (URICOSURIC AGENTS), decrease uric acid production (antihyperuricemics), or alleviate the pain and inflammation of acute attacks of gout. (See all compounds classified as Gout Suppressants.)
M04AA03
M04AA03
M04AA03
M04AA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M04 - Antigout preparations
M04A - Antigout preparations
M04AA - Preparations inhibiting uric acid production
M04AA03 - Febuxostat
Absorption
After oral administration, about 85% of febuxostat is absorbed rapidly. Tmax ranges from 1 to 1.5 hours. Following once-daily oral administration, Cmax was approximately 1.6 0.6 mcg/mL at a dose of 40 mg febuxostat and 2.6 1.7 mcg/mL at a dose of 80 mg febuxostat. A high-fat meal decreased Cmax by 49% and AUC by 18%, but there were no clinically significant changes in the ability of febuxostat to decrease serum uric acid concentrations.
Route of Elimination
Febuxostat is eliminated via both hepatic and renal pathways. Following oral administration of 80 mg radiolabeled febuxostat, approximately 49% of the dose was recovered in the urine. In urine, about 3% of the recovered dose accounted for unchanged febuxostat, 30% accounted for the acyl glucuronide metabolite, 13% accounted for oxidative metabolites and their conjugates, and 3% accounted for unidentified metabolites. Approximately 45% of the total dose was recovered in the feces, where 12% of the dose accounted for the unchanged parent drug. About 1% accounted for the acyl glucuronide metabolite, 25% accounted for oxidative metabolites and their conjugates, and 7% accounted for unidentified metabolites.
Volume of Distribution
The apparent steady-state volume of distribution (Vss/F) of febuxostat ranges from 29 to 75 L, indicating a low to medium volume of distribution.
Clearance
Following oral administration of single doses of 10 to 240 mg, the mean apparent total clearance ranged from 10 to 12 L/h.
Febuxostat is metabolized in the liver by UDP-glucuronosyltransferase (UGT) and Cytochrome P450 (CYP) enzymes, with the relative contribution of each enzyme isoform in the metabolism of febuxostat not fully elucidated. UGT1A1, UGT1A3, UGT1A9, and UGT2B7 mediate conjugation of febuxostat, which approximately accounts for 2244% of the metabolism of the total dose administered, to produce the acyl-glucuronide metabolite. CYP1A2, CYP2C8, CYP2C9, and non-P450 enzymes are responsible for the oxidation reaction, which accounts for 2-8% of the metabolism of the dose. Oxidation reaction produces 67M-1, 67M-2, and 67M-4, which are pharmacologically active metabolites. 67M-1, 67M-2, and 67M-4 can further undergo glucuronidation and sulfation. Hydroxy metabolites are present in human plasma at much lower concentrations than the parent drug.
The apparent mean terminal elimination half-life of approximately 5 to 8 hours.
Gout is a form of acute arthritis that is characterized by the accumulation of crystals of monosodium urate and urate crystals in or around a joint, leading to inflammation and persistent urate crystal deposition in bones, joints, tissues, and other organs that may exacerbate over time. Hyperuricemia is closely related to gout, whereby it may exist for many years before the first clinical attack of gout; thus, aberrated serum uric acid levels and hyperuricemia are believed to be the biochemical aberration involved in the pathogenesis of gout. Xanthine oxidoreductase (XOR) can act as a xanthine oxidase or xanthine dehydrogenase. In humans, it is a critical enzyme for uric acid production as it catalyzes the oxidation reaction steps from hypoxanthine to xanthine and from xanthine to uric acid in the pathway of purine metabolism. Febuxostat potently inhibits XOR, blocking both its oxidase and dehydrogenase activities. With high affinity, febuxostat binds to XOR in a molecular channel leading to the molybdenum-pterin active site, where [allopurinol] demonstrates relatively weak competitive inhibition. XOR is mainly found in the dehydrogenase form under normal physiological conditions; however, in inflammatory conditions, XOR can be converted into the xanthine oxidase form, which catalyzes reactions that produce reactive oxygen species (ROS), such as peroxynitrite. ROS contribute to vascular inflammation and alterations in vascular function. As febuxostat can inhibit both forms of XOR, it can inhibit ROS formation, oxidative stress, and inflammation. In a rat model, febuxostat suppressed renal ischemia-reperfusion injury by attenuating oxidative stress.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
10
PharmaCompass offers a list of Febuxostat API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Febuxostat manufacturer or Febuxostat supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Febuxostat manufacturer or Febuxostat supplier.
PharmaCompass also assists you with knowing the Febuxostat API Price utilized in the formulation of products. Febuxostat API Price is not always fixed or binding as the Febuxostat Price is obtained through a variety of data sources. The Febuxostat Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Febuxostat manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Febuxostat, including repackagers and relabelers. The FDA regulates Febuxostat manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Febuxostat API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Febuxostat manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Febuxostat supplier is an individual or a company that provides Febuxostat active pharmaceutical ingredient (API) or Febuxostat finished formulations upon request. The Febuxostat suppliers may include Febuxostat API manufacturers, exporters, distributors and traders.
click here to find a list of Febuxostat suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Febuxostat DMF (Drug Master File) is a document detailing the whole manufacturing process of Febuxostat active pharmaceutical ingredient (API) in detail. Different forms of Febuxostat DMFs exist exist since differing nations have different regulations, such as Febuxostat USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Febuxostat DMF submitted to regulatory agencies in the US is known as a USDMF. Febuxostat USDMF includes data on Febuxostat's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Febuxostat USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Febuxostat suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Febuxostat Drug Master File in Japan (Febuxostat JDMF) empowers Febuxostat API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Febuxostat JDMF during the approval evaluation for pharmaceutical products. At the time of Febuxostat JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Febuxostat suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Febuxostat Drug Master File in Korea (Febuxostat KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Febuxostat. The MFDS reviews the Febuxostat KDMF as part of the drug registration process and uses the information provided in the Febuxostat KDMF to evaluate the safety and efficacy of the drug.
After submitting a Febuxostat KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Febuxostat API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Febuxostat suppliers with KDMF on PharmaCompass.
A Febuxostat written confirmation (Febuxostat WC) is an official document issued by a regulatory agency to a Febuxostat manufacturer, verifying that the manufacturing facility of a Febuxostat active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Febuxostat APIs or Febuxostat finished pharmaceutical products to another nation, regulatory agencies frequently require a Febuxostat WC (written confirmation) as part of the regulatory process.
click here to find a list of Febuxostat suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Febuxostat as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Febuxostat API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Febuxostat as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Febuxostat and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Febuxostat NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Febuxostat suppliers with NDC on PharmaCompass.
Febuxostat Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Febuxostat GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Febuxostat GMP manufacturer or Febuxostat GMP API supplier for your needs.
A Febuxostat CoA (Certificate of Analysis) is a formal document that attests to Febuxostat's compliance with Febuxostat specifications and serves as a tool for batch-level quality control.
Febuxostat CoA mostly includes findings from lab analyses of a specific batch. For each Febuxostat CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Febuxostat may be tested according to a variety of international standards, such as European Pharmacopoeia (Febuxostat EP), Febuxostat JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Febuxostat USP).

