
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Fedratinib Dihydrochloride Monohydrate
2. Fedratinib Hydrochloride
3. Fedratinib Hydrochloride Monohydrate
4. Inrebic
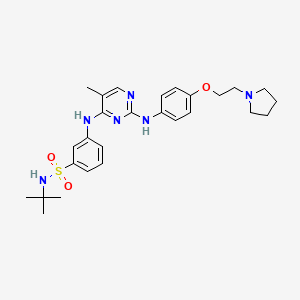
5. N-tert-butyl-3-((5-methyl-2-((4-(2-pyrrolidin-1-ylethoxy)phenyl)amino)pyrimidin-4-yl)amino) Benzenesulfonamide Dihydrochloride Monohydrate
6. N-tert-butyl-3-(5-methyl-2-(4-(2-(pyrrolidin-1-yl)ethoxy)phenylamino) Pyrimidin-4-ylamino)benzenesulfonamide
7. Sar-302503
8. Sar-302503a
9. Sar302503
10. Sar302503a
11. Tg-101348
12. Tg101348
1. 936091-26-8
2. Tg-101348
3. Tg101348
4. Sar302503
5. Sar-302503
6. N-(tert-butyl)-3-((5-methyl-2-((4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)amino)pyrimidin-4-yl)amino)benzenesulfonamide
7. Inrebic
8. Sar 302503
9. Tg 101348
10. Fedratinib (sar302503, Tg101348)
11. N-(1,1-dimethylethyl)-3-[[5-methyl-2-[[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]amino]-4-pyrimidinyl]amino]benzenesulfonamide
12. Tg101348 (sar302503)
13. Chembl1287853
14. 6l1xp550i6
15. 936091-26-8 (free Base)
16. N-tert-butyl-3-(5-methyl-2-(4-(2-(pyrrolidin-1-yl)ethoxy)phenylamino)pyrimidin-4-ylamino)benzenesulfonamide
17. Benzenesulfonamide, N-(1,1-dimethylethyl)-3-((5-methyl-2-((4-(2-(1-pyrrolidinyl)ethoxy)phenyl)amino)-4-pyrimidinyl)amino)-
18. N-tert-butyl-3-(5-methyl-2-(4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino)-pyrimidin-4-ylamino)-benzenesulfonamide
19. N-tert-butyl-3-{[5-methyl-2-({4-[2-(pyrrolidin-1-yl)ethoxy]phenyl}amino)pyrimidin-4-yl]amino}benzenesulfonamide
20. Fedratinib [usan]
21. Fedratinib [usan:inn]
22. Unii-6l1xp550i6
23. C27h36n6o3s
24. 2ta
25. Fedratinib [mi]
26. Fedratinib [inn]
27. Fedratinib (usan/inn)
28. Tg101348(fedratinib)
29. Fedratinib [who-dd]
30. Fedratinib (tg101348)
31. Mls006011155
32. N-tert-butyl-3-[[5-methyl-2-[4-(2-pyrrolidin-1-ylethoxy)anilino]pyrimidin-4-yl]amino]benzenesulfonamide
33. Schembl263741
34. Gtpl5716
35. Chebi:91408
36. Dtxsid90239483
37. Ex-a170
38. Hms3295i03
39. Hms3656l19
40. Hms3744g17
41. Hms3868l03
42. Bcp02300
43. Bdbm50332294
44. Mfcd12922515
45. Nsc767600
46. Nsc800099
47. S2736
48. Zinc19862646
49. Akos015842621
50. Ccg-264990
51. Cs-0052
52. Db12500
53. Ex-5961
54. Nsc-767600
55. Nsc-800099
56. Sb14604
57. Ncgc00244252-01
58. Ncgc00244252-07
59. Ac-30260
60. As-16248
61. Da-40258
62. Hy-10409
63. Smr004702929
64. Db-079623
65. Ft-0705969
66. Ft-0763396
67. Ft-0766818
68. Sw218187-2
69. A25534
70. D10630
71. F17372
72. 091d268
73. J-523769
74. Q7670147
75. Brd-k12502280-001-01-5
76. 945381-69-1
77. N-tert-butyl-3-((5-methyl-2-(4-(2-(pyrrolidin-1-yl)ethoxy)anilino(pyrimidin-4-yl)amino)benzenesulfonamide
78. N-tert-butyl-3-{[5-methyl-2-({4-[2-(pyrrolidin-1-yl)ethoxy]phenyl}amino)pyrimidin-4-yl]amino}benzene-1-sulfonamide
79. Sar302503, N-tert-butyl-3-(5-methyl-2-(4-(2-(pyrrolidin-1-yl)ethoxy)phenylamino)pyrimidin-4-ylamino)benzenesulfonamide
| Molecular Weight | 524.7 g/mol |
|---|---|
| Molecular Formula | C27H36N6O3S |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 11 |
| Exact Mass | 524.25696021 g/mol |
| Monoisotopic Mass | 524.25696021 g/mol |
| Topological Polar Surface Area | 117 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 787 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Fedratinib is indicated to treat adults with primary or secondary myelofibrosis that is either intermediate-2 or high risk.
Inrebic is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis who are Janus Associated Kinase (JAK) inhibitor nave or have been treated with ruxolitinib.
Fedratinib is a kinase inhibitor that inhibits cell division and induces apoptosis. Patients taking fedratinib may experience anemia, thrombocytopenia, gastrointestinal toxicity, hepatic toxicity, or elevated amylase and lipase. These effects should be managed by reducing the dose, temporarily stopping the medication, or providing transfusions on a case by case basis.
L01EJ02
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EJ - Janus-associated kinase (jak) inhibitors
L01EJ02 - Fedratinib
Absorption
A 400mg oral dose results in a Cmax of 1804ng/mL and an AUC of 26,870ng/*hr/mL. Fedratinib has a Tmax of 1.75-3 hours. A high fat breakfast does not significantly affect the absorption of fedratinib.
Route of Elimination
An oral dose of fedratinib is 77% eliminated in the feces with 23% as unchanged drug. 5% is eliminated in the urine, with 3% as unchanged drug.
Volume of Distribution
The apparent volume of distribution is 1770L.
Clearance
The clearance of fedratinib is 13L/h.
Fedratinib is metabolized by CYP3A4, CYP2C19, and flavin-containing monooxygenase 3. Beyond that, data regarding the metabolism of fedratinib is not readily available.
The half life of fedratinib is 41 hours with a terminal half life of 114 hours.
Fedratinib is an inhibitor of Janus Activated Kinase 2 (JAK2) and FMS-like tyrosine kinase 3. JAK2 is highly active in myeloproliferative neoplasms like myelofibrosis. Fedratinib's inhibition of JAK2 inhibits phosphorylation of signal transducer and activator of transcription (STAT) 3 and 5, which prevents cell division and induces apoptosis.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
85
PharmaCompass offers a list of Fedratinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fedratinib manufacturer or Fedratinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fedratinib manufacturer or Fedratinib supplier.
PharmaCompass also assists you with knowing the Fedratinib API Price utilized in the formulation of products. Fedratinib API Price is not always fixed or binding as the Fedratinib Price is obtained through a variety of data sources. The Fedratinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A fedratinib hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of fedratinib hydrochloride, including repackagers and relabelers. The FDA regulates fedratinib hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. fedratinib hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of fedratinib hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A fedratinib hydrochloride supplier is an individual or a company that provides fedratinib hydrochloride active pharmaceutical ingredient (API) or fedratinib hydrochloride finished formulations upon request. The fedratinib hydrochloride suppliers may include fedratinib hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of fedratinib hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A fedratinib hydrochloride DMF (Drug Master File) is a document detailing the whole manufacturing process of fedratinib hydrochloride active pharmaceutical ingredient (API) in detail. Different forms of fedratinib hydrochloride DMFs exist exist since differing nations have different regulations, such as fedratinib hydrochloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A fedratinib hydrochloride DMF submitted to regulatory agencies in the US is known as a USDMF. fedratinib hydrochloride USDMF includes data on fedratinib hydrochloride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The fedratinib hydrochloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of fedratinib hydrochloride suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a fedratinib hydrochloride Drug Master File in Korea (fedratinib hydrochloride KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of fedratinib hydrochloride. The MFDS reviews the fedratinib hydrochloride KDMF as part of the drug registration process and uses the information provided in the fedratinib hydrochloride KDMF to evaluate the safety and efficacy of the drug.
After submitting a fedratinib hydrochloride KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their fedratinib hydrochloride API can apply through the Korea Drug Master File (KDMF).
click here to find a list of fedratinib hydrochloride suppliers with KDMF on PharmaCompass.
fedratinib hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of fedratinib hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right fedratinib hydrochloride GMP manufacturer or fedratinib hydrochloride GMP API supplier for your needs.
A fedratinib hydrochloride CoA (Certificate of Analysis) is a formal document that attests to fedratinib hydrochloride's compliance with fedratinib hydrochloride specifications and serves as a tool for batch-level quality control.
fedratinib hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each fedratinib hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
fedratinib hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (fedratinib hydrochloride EP), fedratinib hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (fedratinib hydrochloride USP).
