
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
API
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
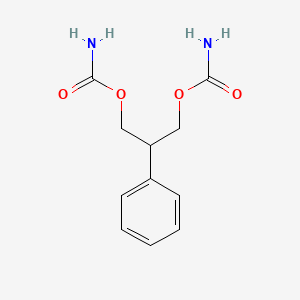

1. (3-carbamoyloxy-2-phenyl-propyl) Carbamate
2. 2 Phenyl 1,3 Propanediol Dicarbamate
3. 2-phenyl-1,3-propanediol Dicarbamate
4. Add 03055
5. Add-03055
6. Add03055
7. Felbamyl
8. Felbatol
9. Taloxa
10. W 554
11. W-554
12. W554
1. 25451-15-4
2. Felbatol
3. 2-phenylpropane-1,3-diyl Dicarbamate
4. 2-phenyl-1,3-propanediol Dicarbamate
5. Taloxa
6. Felbamyl
7. W-554
8. Felbamato
9. Felbamatum
10. 1,3-propanediol, 2-phenyl-, Dicarbamate
11. Add-03055
12. W 554
13. Carbamic Acid 2-phenyltrimethylene Ester
14. Chebi:4995
15. Nsc-759866
16. 3-(carbamoyloxy)-2-phenylpropyl Carbamate
17. Mls000028465
18. X72rbb02n8
19. (3-carbamoyloxy-1,1,3,3-tetradeuterio-2-phenylpropyl) Carbamate
20. Felbamatum [latin]
21. Ncgc00015429-08
22. W-554;add-03055
23. Felbamato [spanish]
24. Smr000058448
25. Dsstox_cid_3041
26. Felbamate [usan:inn]
27. Dsstox_rid_76847
28. Dsstox_gsid_23041
29. Carbamic Acid 3-carbamoyloxy-2-phenyl-propyl Ester
30. (3-carbamoyloxy-2-phenylpropyl) Carbamate
31. Felbatol (tn)
32. Cas-25451-15-4
33. Carbamic Acid, 2-phenyltrimethylene Ester
34. Sr-01000000089
35. Einecs 247-001-4
36. Brn 3345236
37. Unii-x72rbb02n8
38. Hsdb 7525
39. Felbamate Solution
40. Add 03055
41. Mfcd00865296
42. Felbamate (uspinn)
43. Tocris-0869
44. Felbamate [inn]
45. Felbamate [mi]
46. Felbamate [hsdb]
47. Felbamate [usan]
48. 2-phenyl-1,3-propanediol 1,3-dicarbamate
49. Opera_id_1738
50. Prestwick0_000919
51. Felbamate [vandf]
52. Lopac-f-0778
53. Biomol-nt_000203
54. Felbamate [mart.]
55. F 0778
56. Felbamate [usp-rs]
57. Felbamate [who-dd]
58. Chembl1094
59. Lopac0_000524
60. Schembl34947
61. 4-06-00-05993 (beilstein Handbook Reference)
62. Mls001077299
63. Bidd:gt0463
64. Bpbio1_001258
65. Gtpl5473
66. Felbamate [orange Book]
67. Dtxsid9023041
68. Ex-a591
69. Felbamate [usp Monograph]
70. Hms2093p19
71. Hms2234h06
72. Hms3261j09
73. Hms3266l12
74. Hms3370i06
75. Hms3411p21
76. Hms3657i11
77. Hms3675p21
78. Hms3715d20
79. Hms3884e11
80. Pharmakon1600-01505600
81. Bcp27941
82. Hy-b0184
83. Zinc1530803
84. Tox21_110145
85. Tox21_302368
86. Tox21_500524
87. 2-phenyl-1,3-propanedioldicarbamate
88. Bdbm50088430
89. Nsc759866
90. S1330
91. Akos015895100
92. Tox21_110145_1
93. Ac-8197
94. Ccg-204614
95. Cs-2068
96. Db00949
97. Ks-1171
98. Lp00524
99. Nsc 759866
100. Sdccgsbi-0050507.p003
101. Ncgc00015429-01
102. Ncgc00015429-02
103. Ncgc00015429-03
104. Ncgc00015429-04
105. Ncgc00015429-05
106. Ncgc00015429-06
107. Ncgc00015429-07
108. Ncgc00015429-09
109. Ncgc00015429-10
110. Ncgc00015429-11
111. Ncgc00015429-14
112. Ncgc00015429-24
113. Ncgc00023092-02
114. Ncgc00023092-04
115. Ncgc00023092-05
116. Ncgc00023092-06
117. Ncgc00255275-01
118. Ncgc00261209-01
119. Sbi-0050507.p002
120. Db-046702
121. Eu-0100524
122. Ft-0630517
123. Sw197633-2
124. (3-aminocarbonyloxy-2-phenyl-propyl) Carbamate
125. C07501
126. D00536
127. Ab00382985-18
128. Ab00382985_19
129. 451f154
130. A817858
131. Q421301
132. Carbamic Acid (3-carbamoyloxy-2-phenylpropyl) Ester
133. Q-100326
134. Sr-01000000089-2
135. Sr-01000000089-4
136. Sr-01000000089-7
137. Brd-k99107520-001-01-9
138. Brd-k99107520-001-09-2
139. Brd-k99107520-001-18-3
140. Z1541638522
141. Felbamate, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 238.24 g/mol |
|---|---|
| Molecular Formula | C11H14N2O4 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 238.09535693 g/mol |
| Monoisotopic Mass | 238.09535693 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Felbatol |
| PubMed Health | Felbamate (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Felbatol (felbamate) is an antiepileptic available as 400 mg and 600 mg tablets and as a 600 mg/5 mL suspension for oral administration. Its chemical name is 2-phenyl-1,3-propanediol dicarbamate.Felbamate is a white to off-white crystalline powder... |
| Active Ingredient | Felbamate |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 600mg/5ml; 600mg; 400mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Felbamate |
| PubMed Health | Felbamate (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Felbatol (felbamate) is an antiepileptic available as 400 mg and 600 mg tablets and as a 600 mg/5 mL suspension for oral administration. Its chemical name is 2-phenyl-1,3-propanediol dicarbamate.Felbamate is a white to off-white crystalline powder... |
| Active Ingredient | Felbamate |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 600mg/5ml; 600mg; 400mg |
| Market Status | Prescription |
| Company | Amneal Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Felbatol |
| PubMed Health | Felbamate (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Felbatol (felbamate) is an antiepileptic available as 400 mg and 600 mg tablets and as a 600 mg/5 mL suspension for oral administration. Its chemical name is 2-phenyl-1,3-propanediol dicarbamate.Felbamate is a white to off-white crystalline powder... |
| Active Ingredient | Felbamate |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 600mg/5ml; 600mg; 400mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Felbamate |
| PubMed Health | Felbamate (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Felbatol (felbamate) is an antiepileptic available as 400 mg and 600 mg tablets and as a 600 mg/5 mL suspension for oral administration. Its chemical name is 2-phenyl-1,3-propanediol dicarbamate.Felbamate is a white to off-white crystalline powder... |
| Active Ingredient | Felbamate |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 600mg/5ml; 600mg; 400mg |
| Market Status | Prescription |
| Company | Amneal Pharms |
Felbamate is indicated as monotherapy or as an adjunct to other anticonvulsants for the treatment of partial seizures with or without generalization in adults with severe epilepsy that has not responded to other treatment. /Included in US product labe/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Felbamate is indicated as adjunctive therapy in the treatment of partial and generalized seizures associated with Lennox-Gastaut syndrome in children who have not responded to other treatment. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Because use of felbamate has been associated with marked increases in the incidences of aplastic anemia and acute hepatic failure, the manufacturer (Carter-Wallace) in conjunction with FDA warns that the drug should only be initiated or continued in the management of seizures in patients for whom, in the clinician's judgment, the seizure disorder is refractory to alternative safer therapy and is so severe that the benefits of felbamate therapy are believed to outweigh the possible risk of aplastic anemia or acute hepatic failure. For patients already receiving the drug, the likelihood that abrupt withdrawal would pose an even greater risk than that of possible felbamate-associated aplastic anemia or acute hepatic failure also should be considered in the decision to discontinue therapy with the drug. Decisions about the potential benefits and risks of felbamate therapy generally should be made in consultation with appropriate hematologic and hepatic disease experts.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2226
At least 21 reported cases (20 of which occurred in the US) of aplastic anemia have developed in association with felbamate therapy. The rate of aplastic anemia cases currently reported with the drug appears to be at least 40-100 times higher than the expected rate of 2-5 cases per million untreated individuals per year. However, because the onset of felbamate-induced aplastic anemia typically is delayed for weeks to months after initiation of the drug and a substantial fraction of patients had felbamate therapy withdrawn for other reasons prior to this period, the absolute rate of this anemia associated with felbamate probably is higher than the currently reported rate of 1 case per 5000 patients per year. Based on this probability, the manufacturer estimates that the actual risk of aplastic anemia associated with felbamate therapy may be as high as 1 case per 2000 patients (500 cases per million patients) per year or more among those who remain on the drug for longer than a few weeks. While postmarketing surveillance usually captures only a fraction of incident cases, the syndrome is still relatively rare, and no cases were observed during premarket testing in which more than 1600 patients received felbamate therapy. All reports of aplastic anemia associated with felbamate therapy to date have occurred in patients receiving the drug for at least 5 weeks.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2226
Of the 21 patients who developed aplastic anemia while receiving felbamate therapy, 5 (all from the US) have died. While current experience and data are too limited to estimate reliably the fatality rate associated with felbamate-induced aplastic anemia, the estimated case fatality rate for untreated individuals with aplastic anemia from any cause ranges from 20-30%. However, historical fatality rates as high as 70% have been reported for aplastic anemia, and the risk of death secondary to this anemia generally varies with severity and etiology. Although most reported cases have been in white females, risk factors for the development of aplastic anemia in patients receiving felbamate therapy have not been identified. Whether age (range for cases to date: 12-68 years old), gender, or race of the patient, duration of exposure to the drug, dosage, or concomitant use of other anticonvulsant agents or drugs affects the incidence of aplastic anemia in patients receiving felbamate remains to be established. Therefore, the manufacturer recommends that felbamate therapy be discontinued in any patient receiving the drug and alternative therapy initiated as necessary, unless in the clinician's judgment continued felbamate therapy outweighs the risk for aplastic anemia.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2226
Of the 10 patients who developed acute hepatic failure while receiving felbamate therapy, 4 have died, and 1 has received a liver transplant. Whether preexisting hepatic impairment increases the risk of fulminant hepatic failure is unknown; however, the manufacturer recommends that all patients be evaluated for evidence of hepatic impairment prior to initiation of felbamate therapy, and use of the drug is not recommended in patients with preexisting hepatic abnormalities. Other risk factors for the development of acute hepatic failure in patients receiving felbamate have not been identified. Whether age (range for cases to date: 5-78 years old), gender, or race of the patient, duration of exposure to the drug, dosage, or concomitant use of other anticonvulsant agents or drugs affects the incidence of acute hepatic failure in patients receiving felbamate remains to be established.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2226
For more Drug Warnings (Complete) data for 2-PHENYL-1,3-PROPANEDIOL DICARBAMATE (29 total), please visit the HSDB record page.
For use only in those patients who respond inadequately to alternative treatments and whose epilepsy is so severe that a substantial risk of aplastic anemia and/or liver failure is deemed acceptable in light of the benefits conferred by its use.
Felbamate is an antiepileptic indicated as monotherapy or as an adjunct to other anticonvulsants for the treatment of partial seizures resulting from epilepsy. Receptor-binding studies in vitro indicate that felbamate has weak inhibitory effects on GABA-receptor binding, benzodiazepine receptor binding, and is devoid of activity at the MK-801 receptor binding site of the NMDA receptor-ionophore complex. However, felbamate does interact as an antagonist at the strychnine-insensitive glycine recognition site of the NMDA receptor-ionophore complex.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Excitatory Amino Acid Antagonists
Drugs that bind to but do not activate excitatory amino acid receptors, thereby blocking the actions of agonists. (See all compounds classified as Excitatory Amino Acid Antagonists.)
N03AX10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX10 - Felbamate
Absorption
>90%
Volume of Distribution
75682 mL/kg
Clearance
26 +/- 3 mL/hr/kg [single 1200 mg dose]
30 +/- 8 mL/hr/kg [multiple daily doses of 3600 mg]
/Absorption is/ complete (>90%). Absorption is unaffected by food, and both tablet and suspension dosage forms exhibit similar kinetics.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Felbamate enters the central nervous system (CNS), with a brain/plasma coefficient of approximately 0.9. The apparent volume of distribution (Vol D) ranged from 0.73 to 0.85 L per kg of body weight (L/kg) in single and multiple dose studies.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
/Protein binding of felbamate is/ low (20-36%).
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Clearance after a single 1200 mg dose is 26+/- 3 mL/hr/kg, and after multiple daily doses og 3600 mg is 30 +/- 8 mL/hr/kg. ... Felbamate Cmax and AUC are proportionate to dose after single and multiple doses over a range of 100-800 mg single doses and 1200-3600 mg daily doses. Cmin (trough) blood levels are also dose proportionate. ... Felbamate gave dose proportional steady-state peak plasma concentrations in children age 4-12 over a range of 15, 30, and 45 mg/kg/day with peak concentrations of 17, 32, and 49 ug/mL.
Medical Economics Co; Physicians Desk Reference: Generics 2nd ed p.1280 (1996)
For more Absorption, Distribution and Excretion (Complete) data for 2-PHENYL-1,3-PROPANEDIOL DICARBAMATE (8 total), please visit the HSDB record page.
Hepatic
/Biotransformation is/ hepatic, probably by the cytochrome P-450 system; primarily by hydroxylation and conjugation to metabolites that are neither pharmacologically active nor neurotoxic.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
About 40-50% of absorbed dose appears in unchanged in urine, an additional 40% is present as unidentified metabolites and conjugates. About 15% is present parahydroxyfelbamate, 2-hydroxyfelbamate, and felbamate monocarbamate, none of which have significant anticonvulsant activity.
Medical Economics Co; Physicians Desk Reference: Generics 2nd ed p.1280 (1996)
Felbamate (FBM; 2-phenyl-1,3-propanediol dicarbamate) is an approved antiepileptic drug shown to be effective in a variety of seizure disorders refractory to other treatments. However, its use has been restricted because of association with occurrence of rare cases of aplastic anemia and hepatic failure. Since it was shown that FBM metabolism requires glutathione (GSH), we used two experimental protocols to determine if the effects of specific metabolites were sensitive to redox pathways. FBM and its metabolite W873 (2-phenyl-1,3-propanediol monocarbamate), at 0.1 mg/mL, induced increased apoptosis of bone marrow cells from B10.AKM mice as compared with B10.BR mice. Study of the effects of the drug on human promonocytic cell line U937 cells showed that FBM and the metabolite W2986 [2-(4-hydroxyphenyl)-1,3 propanediol dicarbamate], at higher concentrations (0.5 mg/mL), induced apoptosis in this cell line. We also observed that while FBM and its metabolites induced increased apoptosis of B cells with reduced intracellular GSH levels, addition of exogenous GSH decreased apoptosis induced by W873 but did not significantly affect apoptosis induced by FBM or W2986. /The authors/ results suggest that, at concentrations used during the present investigations, FBM metabolites induce apoptosis via redox-sensitive and redox-independent pathways.
PMID:11823110 Husain Z et al; Epilepsy Res 48 (1-2): 57-69 (2002)
Antiepileptic therapy with a broad spectrum drug felbamate (FBM) has been limited due to reports of hepatotoxicity and aplastic anemia associated with its use. It was proposed that a bioactivation of FBM leading to formation of alpha,beta-unsaturated aldehyde, atropaldehyde (ATPAL) could be responsible for toxicities associated with the parent drug. Other members of this class of compounds, acrolein and 4-hydroxynonenal (HNE), are known for their reactivity and toxicity. It has been proposed that the bioactivation of FBM to ATPAL proceeds though a more stable cyclized product, 4-hydroxy-5-phenyltetrahydro-1,3-oxazin-2-one (CCMF) whose formation has been shown recently. Aldehyde dehydrogenase (ALDH) and glutathione transferase (GST) are detoxifying enzymes and targets for reactive aldehydes. This study examined effects of ATPAL and its precursor, CCMF on ALDH, GST and cell viability in liver, the target tissue for its metabolism and toxicity. A known toxin, HNE, which is also a substrate for ALDH and GST, was used for comparison. Interspecies difference in metabolism of FBM is well documented, therefore, human tissue was deemed most relevant and used for these studies. ATPAL inhibited ALDH and GST activities and led to a loss of hepatocyte viability. Several fold greater concentrations of CCMF were necessary to demonstrate a similar degree of ALDH inhibition or cytotoxicity as observed with ATPAL. This is consistent with CCMF requiring prior conversion to the more proximate toxin, ATPAL. GSH was shown to protect against ALDH inhibition by ATPAL. In this context, ALDH and GST are detoxifying pathways and their inhibition would lead to an accumulation of reactive species from FBM metabolism and/or metabolism of other endogenous or exogenous compounds and predisposing to or causing toxicity. Therefore, mechanisms of reactive aldehydes toxicity could include direct interaction with critical cellular macromolecules or indirect interference with cellular detoxification mechanisms.
PMID:12399159 Kapetanovic I et al; Chem Biol Interact 142 (1-2): 119-34 (2002)
20-23 hours
Elimination /half-life is/ 13 to 23 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
The mechanism by which felbamate exerts its anticonvulsant activity is unknown, but in animal test systems designed to detect anticonvulsant activity, felbamate has properties in common with other marketed anticonvulsants. In vitro receptor binding studies suggest that felbamate may be an antagonist at the strychnine-insensitive glycine-recognition site of the N-methyl-D-aspartate (NMDA) receptor-ionophore complex. Antagonism of the NMDA receptor glycine binding site may block the effects of the excitatory amino acids and suppress seizure activity. Animal studies indicate that felbamate may increase the seizure threshold and may decrease seizure spread. It is also indicated that felbamate has weak inhibitory effects on GABA-receptor binding, benzodiazepine receptor binding.
The exact mechanism of action of felbamate is not known, but available data suggest that the drug increases seizure threshold and reduces seizure spread. A predominant effect on any particular cell process has not been demonstrated to date, but felbamate appears to exhibit a spectrum of anticonvulsant activity that is pharmacologically distinct from other currently available agents. In animals, felbamate protects against seizures induced by electrical stimulation, suggesting that it would be effective in the management of tonic-clonic (grand mal) seizures and partial seizures. In animals, felbamate also protects against seizures induced by pentylenetetrazol, indicating that it may be effective in the management of absence (petit mal) seizures. Felbamate also protects against seizures in animals induced by picrotoxin, glutamate, or N-methyl-d, l-aspartic acid; it does not protect against seizures induced by bicuculline or strychnine.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2229
In vitro studies indicate that felbamate has weak inhibitory effects on binding at gamma-aminobutyric acid (GABA) receptors and benzodiazepine receptors. The monocarbamate, p-hydroxy, and 2-hydroxy metabolites of felbamate appear to contribute little, if any, to the anticonvulsant action of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2229
In vitro receptor binding studies suggest that felbamate may be an antagonist at the strychnine-insensitive glycine-recognition site of the N-methyl-D-aspartate (NMDA) receptor-ionophore complex. Antagonism of the NMDA receptor glycine binding site may block the effects of the excitatory amino acids and suppress seizure activity. Animal studies indicate that felbamate may increase the seizure threshold and may decrease seizure spread.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Felbamate is an anticonvulsant used in the treatment of seizures associated with Lennox-Gastaut syndrome and complex partial seizures that are refractory to other medications. Its unique clinical profile is thought to be due to an interaction with N-methyl-D-aspartate (NMDA) receptors, resulting in decreased excitatory amino acid neurotransmission. To further characterize the interaction between felbamate and NMDA receptors, recombinant receptors expressed in Xenopus oocytes were used to investigate the subtype specificity and mechanism of action. Felbamate reduced NMDA- and glycine-induced currents most effectively at NMDA receptors composed of NR1 and NR2B subunits (IC50 = 0.93 mM), followed by NR1-2C (2.02 mM) and NR1-2A (8.56 mM) receptors. The NR1-2B-selective interaction was noncompetitive with respect to the coagonists NMDA and glycine and was not dependent on voltage. Felbamate enhanced the affinity of the NR1-2B receptor for the agonist NMDA by 3.5-fold, suggesting a similarity in mechanism to other noncompetitive antagonists such as ifenprodil. However, a point mutation at position 201 (E201R) of the epsilon2 (mouse NR2B) subunit that affects receptor sensitivity to ifenprodil, haloperidol, and protons reduced the affinity of NR1-epsilon2 receptors for felbamate by only 2-fold. Furthermore, pH had no effect on the affinity of NR1-2B receptors for felbamate. /Investigators/ suggest that felbamate interacts with a unique site on the NR2B subunit (or one formed by NR1 plus NR2B) that interacts allosterically with the NMDA/glutamate binding site. These results suggest that the unique clinical profile of felbamate is due in part to an interaction with the NR1-2B subtype of NMDA receptor.
PMID:10215667 Kleckner N et al; J Pharmacol Exp Ther 289 (2): 886-94 (1999)
The effect of the antiepileptic drug felbamate (FBM) on high-voltage-activated Ca++ currents was studied in cortical and neostriatal neurons acutely isolated from adult rats. Patch-clamp recordings in the whole-cell configuration were performed. Ba++ ions as the charge carrier for Ca++ channels were used. In pyramidal cortical cells, FBM dose-dependently reduced high-voltage-activated Ca++ currents in all the tested neurons. At concentrations of 30 to 100 nM, FBM already produced a significant inhibition of high-voltage-activated Ca++ currents (-6/-15%). At saturating concentrations (1-3 microM), FBM-mediated inhibition averaged 44%. The responses were fully reversible. The dose-response curves revealed IC50 of 504 nM. In striatal neurons, FBM decreased the same conductances by about 28%; the threshold dose was 1 to 2 microM, with an IC50 of 18.7 microM. In both structures, the observed inhibitions were unaffected by omega-conotoxin GVIA and omega-agatoxin IVA, suggesting that N-like channels and P-Like channels were not involved in the FBM-mediated responses. In addition, when omega-conotoxin GVIA and omega-agatoxin IVA (100 nM) were coapplied, the FBM-mediated inhibition on the remaining Ca++ currents averaged 87%. The FBM responses were occluded by micromolar concentrations of nifedipine, supporting a direct interference with dihydropyridine-sensitive channels. It is concluded that the described effect of FBM might represent an efficacious mechanism for either controlling spike discharge from epileptic foci or protecting neurons from excessive Ca++ loading.
PMID:8613908 Stefani A et al; J Pharmacol Exp Ther 277 (1): 121-7 (1996)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
88
PharmaCompass offers a list of Felbamate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Felbamate manufacturer or Felbamate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Felbamate manufacturer or Felbamate supplier.
PharmaCompass also assists you with knowing the Felbamate API Price utilized in the formulation of products. Felbamate API Price is not always fixed or binding as the Felbamate Price is obtained through a variety of data sources. The Felbamate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Felbamate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Felbamate, including repackagers and relabelers. The FDA regulates Felbamate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Felbamate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Felbamate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Felbamate supplier is an individual or a company that provides Felbamate active pharmaceutical ingredient (API) or Felbamate finished formulations upon request. The Felbamate suppliers may include Felbamate API manufacturers, exporters, distributors and traders.
click here to find a list of Felbamate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Felbamate DMF (Drug Master File) is a document detailing the whole manufacturing process of Felbamate active pharmaceutical ingredient (API) in detail. Different forms of Felbamate DMFs exist exist since differing nations have different regulations, such as Felbamate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Felbamate DMF submitted to regulatory agencies in the US is known as a USDMF. Felbamate USDMF includes data on Felbamate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Felbamate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Felbamate suppliers with USDMF on PharmaCompass.
A Felbamate written confirmation (Felbamate WC) is an official document issued by a regulatory agency to a Felbamate manufacturer, verifying that the manufacturing facility of a Felbamate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Felbamate APIs or Felbamate finished pharmaceutical products to another nation, regulatory agencies frequently require a Felbamate WC (written confirmation) as part of the regulatory process.
click here to find a list of Felbamate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Felbamate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Felbamate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Felbamate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Felbamate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Felbamate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Felbamate suppliers with NDC on PharmaCompass.
Felbamate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Felbamate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Felbamate GMP manufacturer or Felbamate GMP API supplier for your needs.
A Felbamate CoA (Certificate of Analysis) is a formal document that attests to Felbamate's compliance with Felbamate specifications and serves as a tool for batch-level quality control.
Felbamate CoA mostly includes findings from lab analyses of a specific batch. For each Felbamate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Felbamate may be tested according to a variety of international standards, such as European Pharmacopoeia (Felbamate EP), Felbamate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Felbamate USP).
