
Synopsis
0
VMF
0
Australia

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Duragesic
2. Durogesic
3. Fentanest
4. Fentanyl Citrate
5. Fentora
6. Phentanyl
7. R 4263
8. R-4263
9. R4263
10. Sublimaze
11. Transmucosal Oral Fentanyl Citrate
1. Phentanyl
2. Fentanil
3. Fentanest
4. Fentora
5. 437-38-7
6. Duragesic
7. Sublimaze
8. Fentanylum
9. Fentanila
10. Sentonil
11. Sublimase
12. Duragesic-100
13. Matrifen
14. Ionsys
15. N-(1-phenethylpiperidin-4-yl)-n-phenylpropionamide
16. Duragesic-12
17. Duragesic-25
18. Duragesic-50
19. Duragesic-75
20. Fentanyl-12
21. Fentanyl-25
22. Fentanyl-50
23. Fentanyl-75
24. Fentanylum [inn-latin]
25. Fentanila [inn-spanish]
26. Fentanyl-100
27. 1-phenethyl-4-n-propionylanilinopiperidine
28. Recuvyra
29. Pecfent
30. Fentanyl Cii
31. N-(1-phenethyl-4-piperidyl)propionanilide
32. N-phenyl-n-[1-(2-phenylethyl)piperidin-4-yl]propanamide
33. N-phenethyl-4-(n-propionylanilino)piperidine
34. 1-phenethyl-4-(n-phenylpropionamido)piperidine
35. R 4263
36. Fendrop
37. Abstral-
38. N-(1-phenethyl-4-piperidinyl)-n-phenylpropionamide
39. Durogesic D-trans
40. N-phenyl-n-(1-(2-phenylethyl)-4-piperidinyl)propanamide
41. N02ab03
42. Chebi:119915
43. N-(1-phenethyl-piperidin-4-yl)-n-phenyl-propionamide
44. Propionanilide, N-(1-phenethyl-4-piperidyl)-
45. Durogesic
46. Chembl596
47. Ids-nf-001
48. R4263
49. En3267
50. Propanamide, N-phenyl-n-(1-(2-phenylethyl)-4-piperidinyl)-
51. Propanamide, N-phenyl-n-[1-(2-phenylethyl)-4-piperidinyl]-
52. Uf599785jz
53. Ad 923
54. Ad-923
55. En-3267
56. Ncgc00168252-01
57. Subsys
58. Fentanil [dcit]
59. Durotep Mt
60. Hsdb 3329
61. Einecs 207-113-6
62. Fentanyl Transdermal System
63. Jns020qd
64. Brn 0494484
65. Unii-uf599785jz
66. Fentanyl [usan:usp:inn:ban]
67. Duragesic (tn)
68. Subsys (tn)
69. Fentanyl-hcl
70. Fentanyl [hsdb]
71. Fentanyl [usan]
72. Fentanyl [inn]
73. Fentanyl [jan]
74. Fentanyl [mi]
75. Fentanyl [vandf]
76. Dsstox_cid_3049
77. Fentanyl [mart.]
78. Epitope Id:153507
79. Fentanyl [who-dd]
80. Schembl8804
81. Fentanyl (jan/usp/inn)
82. Dsstox_rid_76851
83. Fentanyl [ema Epar]
84. Dsstox_gsid_23049
85. Oprea1_152073
86. Oprea1_207148
87. 5-22-08-00049 (beilstein Handbook Reference)
88. Bidd:gt0555
89. Fentanyl [green Book]
90. Fentanyl [ep Impurity]
91. Fentanyl [orange Book]
92. Fentanyl Cii [usp-rs]
93. Gtpl1626
94. Fentanyl [ep Monograph]
95. Dtxsid9023049
96. Fentanyl [usp Monograph]
97. Fentanyl 0.1 Mg/ml In Methanol
98. Fentanyl 1.0 Mg/ml In Methanol
99. N-(1-(2-phenylethyl)-4-piperidinyl)-n-phenylpropanamide
100. Zinc2522669
101. Tox21_112611
102. Bdbm50008984
103. Hy-u00158
104. Pdsp1_000860
105. Pdsp2_000846
106. Fentanyl [ema Epar Veterinary]
107. Cs-7211
108. Db00813
109. Cas-437-38-7
110. N-(1-phenylethyl-4-piperidinyl)propionanilide
111. D00320
112. 1-(2-phenylethyl)-4-(n-propananilido)piperidine
113. L001275
114. Q407541
115. N-phenyl-n-[1-(2-phenylethyl)-4-piperidinyl]propanamide #
116. N-(1-phenethyl-piperidin-4-yl)-n-phenyl-propionamide(fentanyl)
117. Propanamide, N-phenyl-n-(1-(2-phenylethyl)-4-piperidinyl)
118. Fentanyl Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
119. Fentanyl Solution, 100 Mug/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
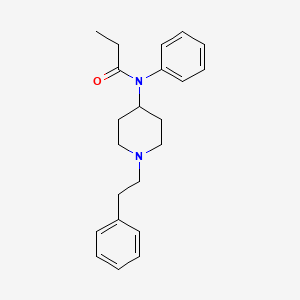
| Molecular Weight | 336.5 g/mol |
|---|---|
| Molecular Formula | C22H28N2O |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 336.220163521 g/mol |
| Monoisotopic Mass | 336.220163521 g/mol |
| Topological Polar Surface Area | 23.6 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 391 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 24 | |
|---|---|
| Drug Name | Duragesic-100 |
| PubMed Health | Fentanyl (Into the mouth) |
| Drug Classes | Analgesic |
| Drug Label | FENTORA (fentanyl buccal tablet) is a potent opioid analgesic, intended for buccal mucosal administration. FENTORA is designed to be placed and retained within the buccal cavity for a period sufficient to allow disintegration of the tablet and absorp... |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 100mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 24 | |
|---|---|
| Drug Name | Duragesic-12 |
| Drug Label | SUBSYS (fentanyl sublingual spray) is a potent opioid analgesic intended for sublingual mucosal administration.SUBSYS is formulated to be sprayed underneath the tongue to allow for absorption of fentanyl across the sublingual mucosa.Active Ingredient... |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 12.5mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 3 of 24 | |
|---|---|
| Drug Name | Duragesic-25 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 25mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 4 of 24 | |
|---|---|
| Drug Name | Duragesic-50 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 50mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 5 of 24 | |
|---|---|
| Drug Name | Duragesic-75 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 75mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 6 of 24 | |
|---|---|
| Drug Name | Fentanyl-100 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 100mcg/hr |
| Market Status | Prescription |
| Company | Watson Labs; Mylan Technologies; Mallinckrodt; Aveva; Lavipharm Labs; Par Pharm |
| 7 of 24 | |
|---|---|
| Drug Name | Fentanyl-12 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 12.5mcg/hr |
| Market Status | Prescription |
| Company | Mylan Technologies |
| 8 of 24 | |
|---|---|
| Drug Name | Fentanyl-25 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 25mcg/hr |
| Market Status | Prescription |
| Company | Watson Labs; Mylan Technologies; Mallinckrodt; Aveva; Lavipharm Labs; Par Pharm |
| 9 of 24 | |
|---|---|
| Drug Name | Fentanyl-50 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 50mcg/hr |
| Market Status | Prescription |
| Company | Watson Labs; Mylan Technologies; Mallinckrodt; Aveva; Lavipharm Labs; Par Pharm |
| 10 of 24 | |
|---|---|
| Drug Name | Fentanyl-75 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 75mcg/hr |
| Market Status | Prescription |
| Company | Watson Labs; Mylan Technologies; Mallinckrodt; Aveva; Lavipharm Labs; Par Pharm |
| 11 of 24 | |
|---|---|
| Drug Name | Fentora |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Tablet |
| Route | Buccal, sublingual |
| Strength | eq 0.1mg base; eq 0.8mg base; eq 0.6mg base; eq 0.4mg base; eq 0.2mg base |
| Market Status | Prescription |
| Company | Cephalon |
| 12 of 24 | |
|---|---|
| Drug Name | Subsys |
| Active Ingredient | Fentanyl |
| Dosage Form | Spray |
| Route | Sublingual |
| Strength | 0.8mg; 1.2mg; 1.6mg; 0.4mg; 0.1mg; 0.6mg; 0.2mg |
| Market Status | Prescription |
| Company | Insys Therap |
| 13 of 24 | |
|---|---|
| Drug Name | Duragesic-100 |
| PubMed Health | Fentanyl (Into the mouth) |
| Drug Classes | Analgesic |
| Drug Label | FENTORA (fentanyl buccal tablet) is a potent opioid analgesic, intended for buccal mucosal administration. FENTORA is designed to be placed and retained within the buccal cavity for a period sufficient to allow disintegration of the tablet and absorp... |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 100mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 14 of 24 | |
|---|---|
| Drug Name | Duragesic-12 |
| Drug Label | SUBSYS (fentanyl sublingual spray) is a potent opioid analgesic intended for sublingual mucosal administration.SUBSYS is formulated to be sprayed underneath the tongue to allow for absorption of fentanyl across the sublingual mucosa.Active Ingredient... |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 12.5mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 15 of 24 | |
|---|---|
| Drug Name | Duragesic-25 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 25mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 16 of 24 | |
|---|---|
| Drug Name | Duragesic-50 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 50mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 17 of 24 | |
|---|---|
| Drug Name | Duragesic-75 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 75mcg/hr |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 18 of 24 | |
|---|---|
| Drug Name | Fentanyl-100 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 100mcg/hr |
| Market Status | Prescription |
| Company | Watson Labs; Mylan Technologies; Mallinckrodt; Aveva; Lavipharm Labs; Par Pharm |
| 19 of 24 | |
|---|---|
| Drug Name | Fentanyl-12 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 12.5mcg/hr |
| Market Status | Prescription |
| Company | Mylan Technologies |
| 20 of 24 | |
|---|---|
| Drug Name | Fentanyl-25 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 25mcg/hr |
| Market Status | Prescription |
| Company | Watson Labs; Mylan Technologies; Mallinckrodt; Aveva; Lavipharm Labs; Par Pharm |
| 21 of 24 | |
|---|---|
| Drug Name | Fentanyl-50 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 50mcg/hr |
| Market Status | Prescription |
| Company | Watson Labs; Mylan Technologies; Mallinckrodt; Aveva; Lavipharm Labs; Par Pharm |
| 22 of 24 | |
|---|---|
| Drug Name | Fentanyl-75 |
| Active Ingredient | Fentanyl |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 75mcg/hr |
| Market Status | Prescription |
| Company | Watson Labs; Mylan Technologies; Mallinckrodt; Aveva; Lavipharm Labs; Par Pharm |
| 23 of 24 | |
|---|---|
| Drug Name | Fentora |
| Active Ingredient | Fentanyl citrate |
| Dosage Form | Tablet |
| Route | Buccal, sublingual |
| Strength | eq 0.1mg base; eq 0.8mg base; eq 0.6mg base; eq 0.4mg base; eq 0.2mg base |
| Market Status | Prescription |
| Company | Cephalon |
| 24 of 24 | |
|---|---|
| Drug Name | Subsys |
| Active Ingredient | Fentanyl |
| Dosage Form | Spray |
| Route | Sublingual |
| Strength | 0.8mg; 1.2mg; 1.6mg; 0.4mg; 0.1mg; 0.6mg; 0.2mg |
| Market Status | Prescription |
| Company | Insys Therap |
Analgesics, Opioid; Adjuvants, Anesthesia; Narcotics; Anesthetics, Intravenous
National Library of Medicine's Medical Subject Headings. Fentanyl. Online file (MeSH, 2017). Available from, as of April 26, 2017: https://www.nlm.nih.gov/mesh/2017/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Fentanyl is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of June 20, 2017: https://clinicaltrials.gov/
Fentanyl citrate is a strong analgesic used preoperatively, during surgery, and in the immediate postoperative period for its analgesic action. In addition, the drug may be used to prevent or relieve tachypnea and postoperative emergence delirium. Fentanyl citrate is used parenterally to provide preoperative anxiolysis and sedation and as a supplement to anesthesia. The drug may be especially useful preoperatively before surgery of short duration or minor surgery in outpatients and in diagnostic procedures or treatments that require the patient to be awake or very lightly anesthetized. Fentanyl citrate may be used as a supplement to general or regional anesthesia, including neuroleptanalgesia in which it is often used in combination with droperidol. When attenuation of the response to surgical stress is especially important, fentanyl citrate may be administered with oxygen and a skeletal muscle relaxant to provide anesthesia without the use of additional anesthetic agents. /Included in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2236
Fentanyl citrate buccal lozenges, buccal tablets, sublingual tablets, and nasal spray, and fentanyl sublingual spray are used for the management of breakthrough pain in patients who are already being treated with, and are tolerant of, opiates used around the clock for persistent cancer pain. /Include in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2237
For more Therapeutic Uses (Complete) data for Fentanyl (11 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: RISK OF RESPIRATORY DEPRESSION. Fatal respiratory depression has occurred in patients treated with oral transmucosal fentanyl citrate, including following use in opioid non-tolerant patients and improper dosing. The substitution of oral transmucosal fentanyl citrate for any other fentanyl product may result in fatal overdose. Due to the risk of respiratory depression, oral transmucosal fentanyl citrate is contraindicated in the management of acute or postoperative pain including headache/migraine and in opioid non-tolerant patients. Death has been reported in children who have accidentally ingested oral transmucosal fentanyl citrate. Oral transmucosal fentanyl citrate must be kept out of reach of children. The concomitant use of oral transmucosal fentanyl citrate with CYP3A4 inhibitors may result in an increase in fentanyl plasma concentrations, and may cause potentially fatal respiratory depression. /Fentanyl citrate lozenge/
US Natl Inst Health; DailyMed. Current Medication Information for Fentanyl Citrate (Fentanyl Citrate) Lozenge (Updated: February 2012). Available from, as of Jun3 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=552ba162-76ed-4bc9-8c2c-fac7a1804da0
/BOXED WARNING/ WARNING: MEDICATION ERRORS. Substantial differences exist in the pharmacokinetic profile of oral transmucosal fentanyl citrate compared to other fentanyl products that result in clinically important differences in the extent of absorption of fentanyl that could result in fatal overdose. When prescribing, do not convert patients on a ug per ug basis from any other fentanyl products to oral transmucosal fentanyl citrate. When dispensing, do not substitute an oral transmucosal fentanyl citrate prescription for other fentanyl products. /Fentanyl citrate lozenge/
US Natl Inst Health; DailyMed. Current Medication Information for Fentanyl Citrate (Fentanyl Citrate) Lozenge (Updated: February 2012). Available from, as of June 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=552ba162-76ed-4bc9-8c2c-fac7a1804da0
/BOXED WARNING/ WARNING: ABUSE POTENTIAL. Oral transmucosal fentanyl citrate contains fentanyl, an opioid agonist and a Schedule II controlled substance, with an abuse liability similar to other opioid analgesics. Oral transmucosal fentanyl citrate can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing oral transmucosal fentanyl citrate in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse or diversion. Because of the risk for misuse, abuse, addiction, and overdose, oral transmucosal fentanyl citrate is available only through a restricted program, required by the Food and Drug Administration, called a Risk Evaluation and Mitigation Strategy (REMS). Under the Transmucosal Immediate Release Fentanyl (TIRF) REMS Access program, outpatients, healthcare professionals who prescribe to outpatients, pharmacies, and distributors must enroll in the program. /Fentanyl citrate lozenge/
US Natl Inst Health; DailyMed. Current Medication Information for Fentanyl Citrate (Fentanyl Citrate) Lozenge (Updated: February 2012). Available from, as of June 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=552ba162-76ed-4bc9-8c2c-fac7a1804da0
/BOXED WARNING/ LIFE-THREATENING RESPIRATORY DEPRESSION. Serious, life-threatening and/or fatal respiratory depression has occurred in patients treated with Abstral, including following use in opioid non-tolerant patients and improper dosing. Monitor for respiratory depression, especially during initiation of Abstral or following a dose increase. The substitution of Abstral for any other fentanyl product may result in fatal overdose.. Due to the risk of respiratory depression, Abstral is contraindicated in the management of acute or postoperative pain including headache/migraine and in opioid non-tolerant patients. /Abstral (fentanyl citrate) tablet, orally disintegrating/
US Natl Inst Health; DailyMed. Current Medication Information for Abstral (Fentanyl Citrate) Tablet, Orally Disintegrating (Updated: December 2016). Available from, as of June 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e60f00e9-2cf4-4c20-b570-1c2ea426c8c7
For more Drug Warnings (Complete) data for Fentanyl (54 total), please visit the HSDB record page.
Minimum Lethal Dosage: Fentanyl 250 ug.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 745
Fentanyl intravenous or intramuscular injections are indicated for short term analgesia during induction, maintenance, and recovery from general or regional anesthesia. These injections are also used with a neuroleptic for premedication, induction, and as an adjunct to maintenance of anesthesia. Finally, fentanyl intravenous or intramuscular injections are used with oxygen for anesthesia in high risk patients. Fentanyl sublingual tablets, transmucosal lozenges, buccal tablets, sublingual sprays, transdermal systems, and nasal sprays are indicated for the management of breakthrough pain in opioid tolerant cancer patients who require around the clock pain management.
FDA Label
PecFent is indicated for the management of breakthrough pain in adults who are already receiving maintenance opioid therapy for chronic cancer pain. Breakthrough pain is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent pain.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone daily, at least 8 mg of oral hydromorphone daily or an equi-analgesic dose of another opioid for a week or longer.
Effentora is indicated for the treatment of breakthrough pain (BTP) in adults with cancer who are already receiving maintenance opioid therapy for chronic cancer pain.
BTP is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent pain.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer.
Ionsys is indicated for the management of acute moderate to severe post-operative pain in adult patients.
For the control of pain associated with orthopaedic and soft tissue surgery in dogs.
Management of acute moderate to severe post-operative pain for use in a hospital setting only
Fentanyl produces strong analgesia through its activation of opioid receptors. It has a duration of action of several hours and a wider therapeutic index as patients develop tolerance to opioids. Fentanyl is associated with a risk of addiction and abuse and should not be mixed with alcohol or benzodiazepines.
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Anesthetics, Intravenous
Ultrashort-acting anesthetics that are used for induction. Loss of consciousness is rapid and induction is pleasant, but there is no muscle relaxation and reflexes frequently are not reduced adequately. Repeated administration results in accumulation and prolongs the recovery time. Since these agents have little if any analgesic activity, they are seldom used alone except in brief minor procedures. (From AMA Drug Evaluations Annual, 1994, p174) (See all compounds classified as Anesthetics, Intravenous.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
N02AB03
N02AB03
N02AB03
QN02AB03
N02AB03
N02AB03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AH - Opioid anesthetics
N01AH01 - Fentanyl
N - Nervous system
N02 - Analgesics
N02A - Opioids
N02AB - Phenylpiperidine derivatives
N02AB03 - Fentanyl
Absorption
Fentanyl sublingual tablets are 54% bioavailable, transmucosal lozenges are 50% bioavailable, buccal tablets are 65% bioavailable, sublingual spray is 76% bioavailable, and nasal spray is 20% more bioavailable than transmucosal (or approximately 64% bioavailable). Fentanyl transmucosal lozenges reach a Cmax of 0.40.1ng/mL for a 200g dose and 2.50.6ng/mL for a 1600g dose with a Tmax of 20-40 minutes. The AUC was 17296ng\*min/mL for a 200g dose and 15081360ng\*min/mL for a 1600g dose. Fentanyl sublingual spray reached a Cmax of 0.200.06ng/mL for a 100g dose and 1.610.60ng/mL for an 800g dose with a Tmax of 0.69-1.25 hours, decreasing as the dose increased. The AUC was 1.250.67ng\*h/mL for a 100g dose and 10.383.70ng\*h/mL for a 800g dose. Fentanyl transdermal systems reached a Cmax of 0.240.20ng/mL with a Tmax of 3.61.3h for a 25g/h dose. The AUC was 0.420.35ng/mL\*h. Fentanyl nasal spray reaches a Cmax of 815301pg/mL with a Tmax of less than 1 hour for a 200g/100L dose. The AUC was 3772pg\*h/mL.
Route of Elimination
Within 72 hours, 75% of a dose of fentanyl is excreted in the urine with <7% unchanged, and 9% is excreted in the feces with <1% unchanged.
Volume of Distribution
The intravenous volume of distribution is 4L/kg (3-8L/kg). The oral volume of distribution is 25.4L/kg. In hepatically impaired patients, the intravenous volume of distribution ranges from 0.8-8L/kg. Fentanyl crosses the blood brain barrier and the placenta.
Clearance
Total plasma clearance of fentanyl is 0.5L/hr/kg (0.3-0.7L/hr/kg) or 42L/hr. Following an intravenous dose, surgical patients displayed a clearance of 27-75L/h, hepatically impaired patients displayed a clearance of 3-80L/h, and renally impaired patients displayed a clearance of 30-78L/h.
Because release of fentanyl from fentanyl transdermal systems and percutaneous permeability of the drug are temperature dependent, serum fentanyl concentrations could theoretically increase by approximately one-third in patients with a body temperature of 40 C. Patients who develop a fever while using fentanyl transdermal system should be observed closely for manifestations of opiate toxicity, and dosage of the drug should be adjusted accordingly. Patients should be cautioned to avoid strenuous exertion that leads to increased core body temperature while wearing the transdermal system. Because application of heat over the fentanyl transdermal system increases mean systemic exposure and peak plasma concentrations of the drug by 120 and 61%, respectively, and has resulted in fatal overdosage, patients wearing a fentanyl transdermal system should be advised to avoid exposing the application site or surrounding area to direct external heat sources.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2243
... The aim of this study was to characterize fentanyl pharmacokinetics in pregnant sheep after intravenous and transdermal dosing during surgical procedure performed to ewe and fetus. Pharmacokinetic parameters reported for non-pregnant sheep and nominal transdermal dose rate were utilized for a priori calculation to achieve analgesic fentanyl concentration (0.5-2 ng/mL) in maternal plasma. A total of 20 Aland landrace ewes at 118-127 gestational days were used. In the first protocol, 1 week before surgery, 10 animals received 2 ug/kg fentanyl intravenous bolus, and on the operation day, transdermal fentanyl patches at nominal dose rate of 2 ug/kg/hr were applied to antebrachium, and ewes were then given a 2 ug/kg intravenous bolus followed by an intra-operative 2.5 ug/kg/hr infusion. In the second protocol, 10 animals received fentanyl only as transdermal patches on the operation day and oxycodone for rescue analgesia. The data were analyzsed with population pharmacokinetic modelling. Intra- and post-operative fentanyl concentrations were similar and slightly lower than the a priori predictions, and elimination and distribution clearances appeared slower during than before or after the surgery. Transdermal patches provided sustained fentanyl absorption for up to 5 days, but the absorption rate was slower than the nominal dose rate and showed a high interindividual variability.
PMID:25626156 Heikkinen EM et al; Basic Clin Pharmacol Toxicol 117 (3): 156-63 (2015)
The influence of the pH of the incubation medium on the cellular accumulation of tritiated fentanyl, lofentanil, and alfentanil was investigated in isolated guinea pig atria. Fentanyl and lofentanil accumulated in atrial tissue up to about 30- and 50-fold, respectively. The amount of drug bound when equilibrium was attained was found to be dependent upon the pH of the medium. By plotting binding equilibria v. pH of the bath, curves were obtained which resembled titration curves. Half-maximal binding was attained at pH values close to the pKa values of fentanyl and lofentanil. Alfentanil was found to accumulate less. The uptake by the tissue was strongly proportional to the extracellular concentration. Atria equilibrated with fentanyl at pH 8.5 released the compound rapidly when exposed to a pH of 7.0, even in the continuous presence of fentanyl in the bath. The consequences of the findings for in vivo conditions are discussed with respect to a possible augmentation of the actions of fentanyl by respiratory acidosis.
PMID:2864047 Lullmann H et al; Br J Anaesth 57 (10): 1012-7 (1985)
Fentanyl is a synthetic opioid agonist used for pain control. Often administered as a transdermal patch, it is an interesting drug for study of postmortem redistribution. We hypothesized that fentanyl concentrations would increase over time after death, as measured in blood drawn on the day prior to autopsy and in blood drawn at the time of autopsy in ten cases where fentanyl patches were identified at the scene. Concentrations were compared, and heart blood to femoral blood ratios were calculated as markers of postmortem redistribution. Fentanyl concentrations measured in peripheral blood drawn the day of autopsy (peripheral blood 2 [PB2]) were higher than those drawn the day prior to autopsy (peripheral blood 1 [PB1]) with a mean ratio (PB2/PB1) of 1.80. The ratio of heart blood concentrations (HB) to femoral blood concentrations drawn at autopsy (PB2) had a mean ratio (HB/PB2) of 1.08. Some cases had blood from the same source analyzed at two different laboratories, and concentrations of fentanyl in those samples showed inter- and intralaboratory differences up to 25 ng/mL. Postmortem fentanyl concentrations may be affected by antemortem factors, postmortem redistribution, and laboratory variability. Forensic pathologists must use caution in interpreting fentanyl levels as part of death investigation.
PMID:25065851 Krinsky CS et al; J Forensic Sci 59 (5): 1275-9 (2014)
For more Absorption, Distribution and Excretion (Complete) data for Fentanyl (32 total), please visit the HSDB record page.
Fentanyl is metabolized to a number of inactive metabolites. Fentanyl is 99% N-dealkylated to norfentanyl by cytochrome P450. It can also be amide hydrolyzed to despropionylfentanyl, or alkyl hydroxylated to hydroxyfentanyl which is N-dealkylated to hydroxynorfentanyl.
Fentanyl does not appear to be metabolized in skin when administered transdermally. Data from clinical studies and from studies using a human keratinocyte cell assay indicate that about 92% of a dose delivered from the fentanyl transdermal system is accounted for as unchanged drug in systemic circulation. Total plasma clearance of fentanyl is reported to be about 500 mL/hour per kg (range: 300-700 mL/hour per kg) or 42-53 L/hour.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2247
Fentanyl citrate is metabolized extensively in the liver and the intestinal mucosa. Animal studies indicate that the drug undergoes oxidation via the microsomal enzymes in the liver and intestinal mucosa (principally cytochrome P-450 [CYP] isoform 3A4) to form norfentanyl; the drug also undergoes hydrolysis to form 4-N-anilinopiperidine and propionic acid. Norfentanyl has been shown to be pharmacologically inactive in animal studies. Fentanyl is excreted in the urine as inactive metabolites and as unchanged drug. Less than 10% of a dose is excreted in urine unchanged and only about 1% is excreted in the feces as unchanged drug.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2247
This study was undertaken to determine if metabolites of fentanyl might be useful in the detection and monitoring of substance abuse. The presence of fentanyl and two of its metabolites in the urine and saliva of seven female patients receiving small doses (110 +/- 56 micrograms) of fentanyl was studied up to 96 hr from the time of administration. Fentanyl and its two metabolites (norfentanyl and despropionylfentanyl) were extracted from samples and analyzed by gas chromatography/mass spectrometry. Unchanged fentanyl was detectable in urine in all patients immediately postoperatively and in 3 of 7 patients at 24 hr. By 72 hr, fentanyl was undetectable. Norfentanyl was present in larger quantities than fentanyl immediately postoperatively and was detected in all patients at 48 hr and in 4 of 7 patients at 96 hr. Despropionylfentanyl was not detected in any of the urine specimens tested. Neither fentanyl nor its metabolites could be detected consistently at any time in saliva. Saliva testing does not appear to be a viable alternative to urine testing based on this study. Urinary norfentanyl might be considered as the substance of choice when testing for fentanyl abuse.
PMID:8452277 Silverstein JH et al; Anesth Analg 76 (3): 618-21 (1993)
Fentanyl has known human metabolites that include Norfentanyl and Phenylacetaldehyde.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half life of fentanyl is 7 hours. The half life of fentanyl sublingual spray is 5-12 hours.
Fentanyl kinetics were studied in patients with cirrhosis and in patients with normal hepatic and renal function undergoing surgery under general anaesthesia, the latter group served as the controls. Plasma fentanyl concentrations declined bi-exponentially in the controls with an average elimination half-life (T1/2 beta) of 263 min; total plasma clearance (Cl) as 10.8 mL/kg/min, and total apparent volume of distribution (V beta) 3.81 L/kg. No significant change was observed in patients with cirrhosis: T1/2 beta was 304 min, Cl 11.3 mL/kg/min and V beta 4.41 L/kg. These data suggest that the elimination half-life of fentanyl is not primarily influenced by the rate at which it is metabolized in the liver.
PMID:7171414 Haberer JP et al; Br J Anaesth 54 (12): 1267-70 (1982)
Fentanyl was administered intravenously and transdermally to eight surgical patients to determine the systemic bioavailability and rate of absorption of the transdermally administered drug. Serum fentanyl concentrations reached a plateau approximately 14 hr after placement of the transdermal fentanyl delivery system. This plateau was maintained until removal of the system at 24 hr. The decline in serum fentanyl concentrations after removal of the transdermal system had a terminal half-life of 17.0 +/- 2.3 hr (mean +/- SD), considerably longer than the terminal elimination half-life seen after intravenous administration of fentanyl in the same patients (6.1 +/- 2.0 hr). ...
PMID:2729633 Varvel JR et al; Anesthesiology 70 (6): 928-34 (1989)
... In order to determine the bioavailability and absorption of fentanyl from OTFC, 12 volunteers were given intravenous fentanyl citrate or OTFC 15 ug/kg on each of two occasions. On a third occasion, the authors assessed oral administration (gastrointestinal absorption) by giving eight of the same volunteers the same dose of a solution of fentanyl citrate to swallow. In each study, arterial blood samples were taken over 24 hr for analysis of plasma fentanyl. After intravenous (iv) administration of fentanyl ... the terminal elimination half-life was 425 +/- 102 min. ...
Streisand JB et al; Anesthesiology 75 (2): 223-9 (1991)
Following IV administration of fentanyl citrate in healthy individuals, the estimated initial distribution half-life was about 6 minutes, the second distribution half-life was about 1 hour, and the terminal half-life was about 16 hours.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2247
Studies of IV fentanyl suggest that clearance of the drug may be decreased and half-life increased in geriatric patients. Although the pharmacokinetic profile of fentanyl in healthy Caucasian adults 65 years of age or older (mean age: 71 years) generally was similar to that in adults 18-45 years of age following application of a fentanyl transdermal system labeled as delivering 100 ug/hour for 72 hours, the mean half-life of the drug was longer in geriatric individuals compared with younger adults (34.4 versus 23.5 hours).
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2247
Fentanyl binds to opioid receptors, especially the mu opioid receptor, which are coupled to G-proteins. Activation of opioid receptors causes GTP to be exchanged for GDP on the G-proteins which in turn down regulates adenylate cyclase, reducing concentrations of cAMP. Reduced cAMP decreases cAMP dependant influx of calcium ions into the cell. The exchange of GTP for GDP results in hyperpolarization of the cell and inhibition of nerve activity.
The aim of the present study was to describe the activity of a set of opioid drugs, including partial agonists, in a cell system expressing only mu opioid receptors. Receptor activation was assessed by measuring the inhibition of forskolin-stimulated cyclic adenosine mono phosphate (cAMP) production. Efficacies and potencies of these ligands were determined relative to the endogenous ligand beta-endorphin and the common mu agonist, morphine. Among the ligands studied naltrexone, WIN 44,441 and SKF 10047, were classified as antagonists, while the remaining ligands were agonists. Agonist efficacy was assessed by determining the extent of inhibition of forskolin-stimulated cAMP production. The rank order of efficacy of the agonists was fentanyl = hydromorphone = beta-endorphin > etorphine = lofentanil = butorphanol = morphine = nalbuphine = nalorphine > cyclazocine = dezocine = metazocine >or= xorphanol. The rank order of potency of these ligands was different from that of their efficacies; etorphine > hydromorphone > dezocine > xorphanol = nalorphine = butorphanol = lofentanil > metazocine > nalbuphine > cyclazocine > fentanyl > morphine >>>> beta-endorphin. These results elucidate the relative activities of a set of opioid ligands at mu opioid receptor and can serve as the initial step in a systematic study leading to understanding of the mode of action of opioid ligands at this receptor. Furthermore, these results can assist in understanding the physiological effect of many opioid ligands acting through mu opioid receptors.
PMID:12513698 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC140036 Gharagozlou P et al; BMC Pharmacol 3: 1 (2003)
Diabetic neuropathy is one of the most frequent complications of diabetes mellitus. Therefore, the present study was designed to investigate the anti-hyperalgesic mechanism of fentanyl in a mouse model of streptozotocin-induced diabetic neuropathy. The antinociceptive response was assessed by recording the latency in a tail-flick test. The tail-flick latency in diabetic mice was significantly shorter than that in non-diabetic mice. Fentanyl, at doses of 3 and 10 ug/kg, s.c., produced a dose-dependent increase in the tail-flick latencies in diabetic mice. While fentanyl (3 ug/kg, s.c.) did not produce a significant inhibition of the tail-flick response in non-diabetic mice, it significantly prolonged the tail-flick latency in diabetic mice to the same level as the baseline latency in non-diabetic mice. Although pretreatment with naloxone (3 mg/kg, s.c.) completely antagonized fentanyl-induced antinociception in non-diabetic mice, it had no effect on the antinociceptive effect of fentanyl in diabetic mice. Pretreatment with either of the voltage-gated sodium channel openers fenvarelarte and veratridine practically abolished the antinociceptive effects of fentanyl in diabetic mice. However, neither fenvarelate nor veratridine affected the antinociceptive effect of fentanyl in non-diabetic mice. These results suggest that the anti-hyperalgesic effect of fentanyl is mediated through the blockade of sodium channels in diabetic mice, whereas opioid receptors mediate the antinociceptive effect of fentanyl in non-diabetic mice.
PMID:24704555 Tanaka K et al; Eur J Pharmacol 733: 68-74 (2014)
Tolerance to opioids frequently follows repeated drug administration and affects the clinical utility of these analgesics. Studies in simple cellular systems have demonstrated that prolonged activation of opioid receptors produces homologous receptor desensitization by G-protein receptor kinase mediated receptor phosphorylation and subsequent beta-arrestin binding. To define the role of this regulatory mechanism in the control of the electrophysiological and behavioral responses to opioids, we used mice having a targeted disruption of the G-protein receptor kinase 3 (GRK3) gene. Mice lacking GRK3 did not differ from wild-type littermates neither in their response latencies to noxious stimuli on the hot-plate test nor in their acute antinociceptive responses to fentanyl or morphine. Tolerance to the electrophysiological response to the opioid fentanyl, measured in vitro in the hippocampus, was blocked by GRK3 deletion. In addition, tolerance to the antinociceptive effects of fentanyl was significantly reduced in GRK3 knockouts compared to wild-type littermate controls. Tolerance to the antinociceptive effects of morphine was not affected by GRK3 deletion although morphine tolerance in hippocampal slices from GRK3 knockout mice was significantly inhibited. Tolerance developed more slowly in vitro to morphine than fentanyl supporting previous work in in vitro systems showing a correlation between agonist efficacy and GRK3-mediated desensitization. The results of these studies suggest that GRK3-mediated mechanisms are important components of both electrophysiologic and behavioral opioid tolerance. Fentanyl, a high efficacy opioid, more effectively produced GRK3-dependent effects than morphine, a low efficacy agonist.
PMID:14662727 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1574178 Terman GW et al; Br J Pharmacol 141 (1):55-64 (2004)
G protein-coupled receptor desensitization is typically mediated by receptor phosphorylation by G protein-coupled receptor kinase (GRK) and subsequent arrestin binding; morphine, however, was previously found to activate a c-Jun N-terminal kinase (JNK)-dependent, GRK/arrestin-independent pathway to produce mu opioid receptor (MOR) inactivation in spinally-mediated, acute anti-nociceptive responses. In the current study, we determined that JNK2 was also required for centrally-mediated analgesic tolerance to morphine using the hotplate assay. We compared JNK activation by morphine and fentanyl in JNK1(-/-), JNK2(-/-), JNK3(-/-), and GRK3(-/-) mice and found that both compounds specifically activate JNK2 in vivo; however, fentanyl activation of JNK2 was GRK3-dependent, whereas morphine activation of JNK2 was GRK3-independent. In MOR-GFP expressing HEK293 cells, treatment with either arrestin siRNA, the Src family kinase inhibitor PP2, or the protein kinase C (PKC) inhibitor Go6976 indicated that morphine activated JNK2 through an arrestin-independent Src- and PKC-dependent mechanism, whereas fentanyl activated JNK2 through a Src-GRK3/arrestin-2-dependent and PKC-independent mechanism. This study resolves distinct ligand-directed mechanisms of JNK activation by mu opioid agonists and understanding ligand-directed signaling at MOR may improve opioid therapeutics.
PMID:26056051 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4492890 Kuhar JR et al; Cell Signal 27 (9): 1799-806 (2015)
Opioids are the most effective and widely used drugs in the treatment of severe acute and chronic pain. They act through opioid receptors that belong to the family of G protein-coupled receptors. Three classes of opioid receptors (mu, delta, kappa), expressed in the central and peripheral nervous system, have been identified. The analgesic effect of opioids is mediated through multiple pathways of opioid receptor signaling (e.g., G(i/o) coupling, cAMP inhibition, Ca(++) channel inhibition). The standard exogenous opioid analgesics used in the operating room are fentanyl, sufentanil, morphine, alfentanil, and remifentanil.
PMID:18685878 Zollner C, Schafer M; Anaesthesist 57 (7): 729-40 (2008)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
35
PharmaCompass offers a list of Fentanyl API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fentanyl manufacturer or Fentanyl supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fentanyl manufacturer or Fentanyl supplier.
PharmaCompass also assists you with knowing the Fentanyl API Price utilized in the formulation of products. Fentanyl API Price is not always fixed or binding as the Fentanyl Price is obtained through a variety of data sources. The Fentanyl Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fentanyl manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fentanyl, including repackagers and relabelers. The FDA regulates Fentanyl manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fentanyl API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fentanyl manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fentanyl supplier is an individual or a company that provides Fentanyl active pharmaceutical ingredient (API) or Fentanyl finished formulations upon request. The Fentanyl suppliers may include Fentanyl API manufacturers, exporters, distributors and traders.
click here to find a list of Fentanyl suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fentanyl DMF (Drug Master File) is a document detailing the whole manufacturing process of Fentanyl active pharmaceutical ingredient (API) in detail. Different forms of Fentanyl DMFs exist exist since differing nations have different regulations, such as Fentanyl USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fentanyl DMF submitted to regulatory agencies in the US is known as a USDMF. Fentanyl USDMF includes data on Fentanyl's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fentanyl USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fentanyl suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Fentanyl Drug Master File in Japan (Fentanyl JDMF) empowers Fentanyl API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Fentanyl JDMF during the approval evaluation for pharmaceutical products. At the time of Fentanyl JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Fentanyl suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Fentanyl Drug Master File in Korea (Fentanyl KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Fentanyl. The MFDS reviews the Fentanyl KDMF as part of the drug registration process and uses the information provided in the Fentanyl KDMF to evaluate the safety and efficacy of the drug.
After submitting a Fentanyl KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Fentanyl API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Fentanyl suppliers with KDMF on PharmaCompass.
A Fentanyl CEP of the European Pharmacopoeia monograph is often referred to as a Fentanyl Certificate of Suitability (COS). The purpose of a Fentanyl CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Fentanyl EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Fentanyl to their clients by showing that a Fentanyl CEP has been issued for it. The manufacturer submits a Fentanyl CEP (COS) as part of the market authorization procedure, and it takes on the role of a Fentanyl CEP holder for the record. Additionally, the data presented in the Fentanyl CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Fentanyl DMF.
A Fentanyl CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Fentanyl CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Fentanyl suppliers with CEP (COS) on PharmaCompass.
A Fentanyl written confirmation (Fentanyl WC) is an official document issued by a regulatory agency to a Fentanyl manufacturer, verifying that the manufacturing facility of a Fentanyl active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Fentanyl APIs or Fentanyl finished pharmaceutical products to another nation, regulatory agencies frequently require a Fentanyl WC (written confirmation) as part of the regulatory process.
click here to find a list of Fentanyl suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Fentanyl as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Fentanyl API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Fentanyl as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Fentanyl and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Fentanyl NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Fentanyl suppliers with NDC on PharmaCompass.
Fentanyl Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fentanyl GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fentanyl GMP manufacturer or Fentanyl GMP API supplier for your needs.
A Fentanyl CoA (Certificate of Analysis) is a formal document that attests to Fentanyl's compliance with Fentanyl specifications and serves as a tool for batch-level quality control.
Fentanyl CoA mostly includes findings from lab analyses of a specific batch. For each Fentanyl CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fentanyl may be tested according to a variety of international standards, such as European Pharmacopoeia (Fentanyl EP), Fentanyl JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fentanyl USP).
