
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
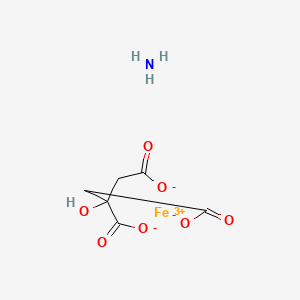

1. 1185-57-5
2. Ammonium Iron(iii) Citrate
3. Ferric Ammonium Citrate, Brown
4. Mfcd00013099
5. Iron(3+) 2-hydroxypropane-1,2,3-tricarboxylate Ammoniate
6. Ferriseltz (tn)
7. 1,2,3-propanetricarboxylic Acid, 2-hydroxy-, Ammonium Iron Salt
8. 7050-19-3
9. Ammonium Iron Iii Citrate
10. Ammonium Ferric Citrate;fac
11. Schembl1920826
12. Ammonium-iron(iii) Citrate, Brown
13. Ferric Ammonium Citrate (jan/usp)
14. Akos015918211
15. D01644
16. E75831
17. J-003847
18. Azane;2-hydroxypropane-1,2,3-tricarboxylate;iron(3+)
| Molecular Weight | 261.98 g/mol |
|---|---|
| Molecular Formula | C6H8FeNO7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 261.965012 g/mol |
| Monoisotopic Mass | 261.965012 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 211 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Source of iron in treating iron-deficiency anemias. It is less constipating than inorg forms of iron. It is free from astringent & irritant properties. However, ferric ion is less well absorbed than ferrous ion, so that its supposed advantages are outweighed by its lesser efficacy, and it is considered to be an obsolete preparation. In the forms presently marketed, a unit dose provides only the recommended daily allowance of iron (15 mg). /former use/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 777
VET: Used in prevention & treatment of anemias. Oral biololgy availabilities in rats and chicks have been reported as 107 and 115% respectively, compared to ferrous sulfate. Counteracts oral poisonous effects of gossypol (in cottonseed meal) in poultry trials. Used in wide variety of oral and parenteral hematinics.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 216
Hematinic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 90
VET: In iron deficiency anemia.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 90
For more Therapeutic Uses (Complete) data for FERRIC AMMONIUM CITRATE (6 total), please visit the HSDB record page.
V - Various
V08 - Contrast media
V08C - Magnetic resonance imaging contrast media
V08CA - Paramagnetic contrast media
V08CA07 - Ferric ammonium citrate
The absorption and endogenous excretion of iron in man was studied by monitoring the fecal excretion of a stable iron isotope (58Fe). The study was carried out for 12 healthy volunteers who were divided into two groups. Group I received 58Fe-labeled ferric ammonium citrate (III) (58FeAC) equivalent to 6 mg of iron as a control, and group II received a combination of 500 mg of vitamin C and 58FeAC. A new formula was used to calculate the 58Fe absorption ratio reflecting the pool of iron in the intestinal cells, and the ratio was compared with that obtained from Janghorbani's formula, which has been used as one of the common methods. As a result, the 58Fe absorption ratio in group II was statistically significantly higher than that of group I (34.4 +/- 6.1% vs. 15.0 +/- 5.5%, M +/- SD) using Janghorbani's formula. The similar absorption ratio (34.1 +/- 6.0% vs. 14.8 +/- 5.5%) was also obtained by our new formula. Our results confirmed the previous findings that the availability of iron is stimulated by the supplementation of vitamin C. Both formulae agreed in the absorption of iron, indicating that the endogenous excretion of iron (caused by the desquamated cells) in the intestine does not disguise the iron absorption.
PMID:1294703 Hashimoto F et al; J Nutr Sci Vitaminol (Tokyo) 38 (5): 435-49 (1992)
The absorption of a commercial brand of small-particle reduced iron was evaluated in 10 normal subjects. For each subject, the hemoglobin incorporation method was used to measure the true absorption of 60 mg of iron from either ferrous sulfate or ferric ammonium citrate. The iron tolerance test (ITT) was also studied for these two compounds and for reduced iron. This procedure consisted of measuring the area under the curve of plasma iron elevations at specified times for 6 hours, or the peak plasma iron, corrected by the plasma iron disappearance rate obtained from measuring plasma iron at specified times for 4 hours after the slow intravenous injection of 0.4 mg of iron as ferric citrate. Only the ITT was used to measure the absorption of 60 mg of reduced iron. Reference dose iron ascorbate absorption was measured in each subject. The absorption of ferric ammonium citrate and reduced iron was expressed as percent of dose and also as absorption percent of that of ferrous sulfate. Mean % geometric "true absorptions" were 39.0 for reference dose, 10.4 for FeSO4 and 2.4 for ferric ammonium citrate. The later was 23% that of FeSO4. By ITT the mean geometric % absorptions were 7.9, 3.7 and 3.2 for FeSO4, ferric ammonium citrate and reduced iron respectively, or 47 and 41% of that of FeSO4. We propose that the true absorption of the commercial brand of reduced iron tested was 20% that of FeSO4 based on the relation between the ITT results of reduced iron and the ITT and true absorption values of ferric ammonium citrate in relation to FeSO4.
PMID:11791474 Gonzalez H et al; Arch Latinoam Nutr 51 (3): 217-24 (2001)
... Iron loading by 24-hour incubation with 0.36 mmol/L ferric ammonium citrate resulted in a decrease in the activity of nicotinamide adenine dinucleotide (NADH)-cytochrome c oxidoreductase (complex I+III) to 35.3%+/-11.2% of the value in untreated controls; of succinate-cytochrome c oxidoreductase (complex II+III) to 57.4%+/-3.1%; and of succinate dehydrogenase to 63.5%+/-12.6% (p < 0.001 in all cases). The decrease in activity of other mitochondrial enzymes, including NADH-ferricyanide reductase, succinate ubiquinone oxidoreductase (complex II), cytochrome c oxidase (complex IV), and ubiquinol cytochrome c oxidoreductase (complex III), was less impressive and ranged from 71.5%+/-15.8% to 91.5%+/-14.6% of controls. That the observed loss of respiratory enzyme activity was a specific effect of iron toxicity was clearly demonstrated by the complete restoration of enzyme activities by in vitro iron chelation therapy. Sequential treatment with iron and doxorubicin caused a loss of complex I+III and complex II+III activity that was greater than that seen with either agent alone but was only partially correctable by DF treatment. Alterations in cellular adenosine triphosphate measurements paralleled very closely the changes observed in respiratory complex activity.
PMID:9605112 Link G et al; J Lab Clin Med 131 (5): 466-74 (1998)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
47
PharmaCompass offers a list of Ferric Ammonium Citrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ferric Ammonium Citrate manufacturer or Ferric Ammonium Citrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ferric Ammonium Citrate manufacturer or Ferric Ammonium Citrate supplier.
PharmaCompass also assists you with knowing the Ferric Ammonium Citrate API Price utilized in the formulation of products. Ferric Ammonium Citrate API Price is not always fixed or binding as the Ferric Ammonium Citrate Price is obtained through a variety of data sources. The Ferric Ammonium Citrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ferric Ammonium Citrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ferric Ammonium Citrate, including repackagers and relabelers. The FDA regulates Ferric Ammonium Citrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ferric Ammonium Citrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ferric Ammonium Citrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ferric Ammonium Citrate supplier is an individual or a company that provides Ferric Ammonium Citrate active pharmaceutical ingredient (API) or Ferric Ammonium Citrate finished formulations upon request. The Ferric Ammonium Citrate suppliers may include Ferric Ammonium Citrate API manufacturers, exporters, distributors and traders.
click here to find a list of Ferric Ammonium Citrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ferric Ammonium Citrate DMF (Drug Master File) is a document detailing the whole manufacturing process of Ferric Ammonium Citrate active pharmaceutical ingredient (API) in detail. Different forms of Ferric Ammonium Citrate DMFs exist exist since differing nations have different regulations, such as Ferric Ammonium Citrate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ferric Ammonium Citrate DMF submitted to regulatory agencies in the US is known as a USDMF. Ferric Ammonium Citrate USDMF includes data on Ferric Ammonium Citrate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ferric Ammonium Citrate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ferric Ammonium Citrate suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ferric Ammonium Citrate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ferric Ammonium Citrate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ferric Ammonium Citrate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ferric Ammonium Citrate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ferric Ammonium Citrate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ferric Ammonium Citrate suppliers with NDC on PharmaCompass.
Ferric Ammonium Citrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ferric Ammonium Citrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ferric Ammonium Citrate GMP manufacturer or Ferric Ammonium Citrate GMP API supplier for your needs.
A Ferric Ammonium Citrate CoA (Certificate of Analysis) is a formal document that attests to Ferric Ammonium Citrate's compliance with Ferric Ammonium Citrate specifications and serves as a tool for batch-level quality control.
Ferric Ammonium Citrate CoA mostly includes findings from lab analyses of a specific batch. For each Ferric Ammonium Citrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ferric Ammonium Citrate may be tested according to a variety of international standards, such as European Pharmacopoeia (Ferric Ammonium Citrate EP), Ferric Ammonium Citrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ferric Ammonium Citrate USP).
