
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
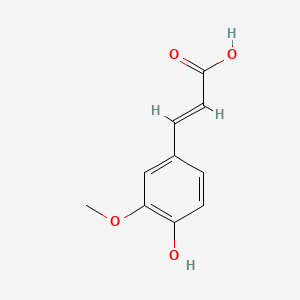

1. 3-(4-hydroxy-3-methoxyphenyl)-2-propenoic Acid
2. 4-hydroxy-3-methoxycinnamic Acid
3. 8,8'-diferulic Acid
4. Cis-ferulic Acid
5. Ferulic Acid, (e)-isomer
6. Ferulic Acid, (z)-isomer
7. Ferulic Acid, Monosodium Salt
8. Sodium Ferulate
9. Trans-ferulic Acid
1. Trans-ferulic Acid
2. 1135-24-6
3. 537-98-4
4. 4-hydroxy-3-methoxycinnamic Acid
5. Trans-4-hydroxy-3-methoxycinnamic Acid
6. 3-(4-hydroxy-3-methoxyphenyl)acrylic Acid
7. (e)-ferulic Acid
8. Ferulate
9. Coniferic Acid
10. 2-propenoic Acid, 3-(4-hydroxy-3-methoxyphenyl)-
11. 3-(4-hydroxy-3-methoxyphenyl)-2-propenoic Acid
12. Ferulic Acid, Trans-
13. Fumalic Acid
14. (e)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoic Acid
15. (2e)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic Acid
16. Cinnamic Acid, 4-hydroxy-3-methoxy-
17. 3-methoxy-4-hydroxycinnamic Acid
18. (e)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic Acid
19. Cinnamic Acid, 4-hydroxy-3-methoxy-, (e)-
20. Mfcd00004400
21. (e)-4-hydroxy-3-methoxycinnamic Acid
22. 2-propenoic Acid, 3-(4-hydroxy-3-methoxyphenyl)-, (2e)-
23. (e)-4'-hydroxy-3'-methoxycinnamic Acid
24. (2e)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoic Acid
25. Cinnamic Acid, 4-hydroxy-3-methoxy-, Trans-
26. 2-propenoic Acid, 3-(4-hydroxy-3-methoxyphenyl)-, (e)-
27. Avm951zwst
28. (e)-3-(4-hydroxy-3-methoxyphenyl)acrylic Acid
29. 97274-61-8
30. (2e)-3-(4-hydroxy-3-methoxyphenyl)acrylic Acid
31. Ferulic Acid Dehydrogenation Homopolymer
32. Fumalic Acid (ferulic Acid)
33. 4-hydroxy-3-methoxycinnamate
34. Chembl32749
35. 3-(4-hydroxy-3-methoxyphenyl)-2-propenoic Acid Homopolymer
36. Chebi:17620
37. Nsc2821
38. Nsc-2821
39. 3-methoxy-4-hydroxy-trans-cinnamate
40. Nsc-51986
41. (e)-3-(4-hydroxy-3-methoxy-phenyl)prop-2-enoic Acid
42. (e)-ferulate
43. 3-(4-hydroxy-3-methoxyphenyl)propenoic Acid
44. Trans-ferulic Acid (purified By Sublimation)
45. Cinnamic Acid,4-hydroxy,3-methoxy Ferulic Acid
46. Caffeic Acid 3-methyl Ether
47. 2-propenoic Acid, 3-(4-hydroxy-3-methoxyphenyl)-, Homopolymer
48. 3-methoxy-4-hydroxy-trans-cinnamic Acid
49. Smr000112202
50. 4-hydroxy-3-methoxy Cinnamic Acid
51. Einecs 208-679-7
52. Unii-avm951zwst
53. Ferulic Acid (trans-4-hydroxy-3-methoxycinnamic Acid)
54. Ferulasaure
55. Trans-ferulate
56. (e)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoate
57. Ccris 3256
58. Ccris 7127
59. Ccris 7575
60. Hsdb 7663
61. Trans-ferulicacid
62. Nsc-674320
63. Nsc 2821
64. Ferulic Acid, E-
65. Einecs 214-490-0
66. Nsc 51986
67. (e)-coniferic Acid
68. Trans-4-hydroxy-3-methoxycinnamicacid
69. Ferulic Acid (m5)
70. Nsc 674320
71. Ferulic Acid ,(s)
72. Ferulic-acid
73. Ferulic Acid, Synthetic
74. Spectrum5_000554
75. Bmse000459
76. Bmse000587
77. Bmse010211
78. Ferulic Acid [mi]
79. Trans-ferulic Acid, 99%
80. Ferulic Acid [hsdb]
81. Ferulic Acid [inci]
82. Schembl15673
83. Bspbio_003168
84. Mls001066385
85. Mls001332483
86. Mls001332484
87. Mls002207079
88. Mls006011435
89. Spectrum1501017
90. Trans-ferulic Acid, >=99%
91. Ferulic Acid [usp-rs]
92. Ferulic Acid [who-dd]
93. Zinc58258
94. Dtxsid70892035
95. Hms1921d05
96. Hms2269p04
97. (e)-4-hydroxy-3-methoxycinnamate
98. Trans-4-hydroxy-3-methoxycinnamate
99. Albb-013505
100. Bcp21231
101. Bcp21789
102. Hy-n0060
103. Nsc51986
104. Str00961
105. (e)-4-hydroxy-3-methoxy-cinnamate
106. Trans-ferulic Acid [who-dd]
107. (e)4-hydroxy-3-methoxycinnamic Acid
108. Ac7905
109. Bbl010345
110. Bdbm50214744
111. Ccg-38860
112. S2300
113. Stk801551
114. Akos000263735
115. Ac-7965
116. Bcp9000163
117. Db07767
118. Ps-3435
119. Sdccgmls-0066667.p001
120. Trans-3-methoxy-4-hydroxycinnamic Acid
121. (e)-4-hydroxy-3-methoxy-cinnamic Acid
122. 3-(4-hydroxy-3-methoxyphenyl)propenoate
123. 4-hydroxy-3-methoxycinnamic Acid, Trans
124. Ncgc00094889-01
125. Ncgc00094889-02
126. Ncgc00094889-03
127. Ncgc00094889-04
128. Ac-10321
129. Bs-17543
130. Smr004703246
131. Am20060784
132. Cs-0007108
133. F1257
134. H0267
135. N1878
136. Sw219616-1
137. C01494
138. A829775
139. Q417362
140. Sr-01000765539
141. (2e)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoate
142. J-002980
143. Sr-01000765539-3
144. (e)-3-(3-methoxy-4-oxidanyl-phenyl)prop-2-enoic Acid
145. 3-(4-hydroxy-3-methoxyphenyl)prop-2-enoicacid
146. Ferulic Acid (constituent Of Black Cohosh) [dsc]
147. 055e203f-b305-4b7f-8ce7-f9c0c03ab609
148. 3986a1be-a670-4b06-833b-e17253079fd8
149. Ferulic Acid, European Pharmacopoeia (ep) Reference Standard
150. Trans-ferulic Acid, Certified Reference Material, Tracecert(r)
151. Diethyl2-(acetamido)-2-(2-(bromomethyl)-5-nitrobenzyl)malonate
152. Ferulic Acid, United States Pharmacopeia (usp) Reference Standard
153. Trans-ferulic Acid, Matrix Substance For Maldi-ms, >=99.0% (hplc)
154. 4-hydroxy-3-methoxycinnamic Acid, Mixture Of Isomers, Analytical Reference Material
155. Ferulic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
156. 831-85-6
| Molecular Weight | 194.18 g/mol |
|---|---|
| Molecular Formula | C10H10O4 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 194.05790880 g/mol |
| Monoisotopic Mass | 194.05790880 g/mol |
| Topological Polar Surface Area | 66.8 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 224 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ferulic acid (FA) is an effective scavenger of free radicals and it has been approved in certain countries as food additive to prevent lipid peroxidation.
PMID:18188410 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2127228 Srinivasan M et al; J Clin Biochem Nutr 40 (2): 92-100 (2007)
Sodium ferulate (SF) or 3-methoxy-4-hydroxy-cinamate sodium is an active principle from Angelica sinensis, Cimicifuga heracleifolia, Lignsticum chuangxiong, and other plants. It has been used in traditional Chinese medicine and is approved by State Drugs Administration of China as a drug for treatment of cardiovascular and cerebrovascular diseases. SF has antithrombotic, platelet aggregation inhibitory and antioxidant activities in animals and humans. For several decades SF has been widely used in China to treat cardiovascular and cerebrovascular diseases and to prevent thrombosis... /Sodium ferulate/
PMID:16007232 Wang BH, Ou-Yang JP; Cardiovasc Drug Rev 23 (2): 161-72 (2005)
/EXPL THER/ Ligusticum Chuanxiong and its effective components were studied in the treatment of ischemic stroke, a common emergent disease in China. Some injections of the medicines, including Ligusticum, Ligustrazine, Ligustylid and ferulic acid, were tested clinically and experimentally. The results showed that the effects of the drugs were the same as or even better than those of the controls, such as papaverine, dextran and aspirin-persantin. They could improve brain microcirculation through inhibiting thrombus formation and platelet aggregation as well as blood viscosity.
PMID:1291208 Chen KJ, Chen K; Chin Med J (Engl). 105 (10): 870-3 (1992)
/EXPL THER/ Although more definitive research is necessary, several natural therapies show promise in treating hot flashes without the risks associated with conventional therapies. Soy and other phytoestrogens, black cohosh, evening primrose oil, vitamin E, the bioflavonoid hesperidin with vitamin C, ferulic acid, acupuncture treatment, and regular aerobic exercise have been shown effective in treating hot flashes in menopausal women.
PMID:12946239 Philip HA; Altern Med Rev 8 (3): 284-302 (2003)
For more Therapeutic Uses (Complete) data for FERULIC ACID (6 total), please visit the HSDB record page.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Free Radical Scavengers
Substances that eliminate free radicals. Among other effects, they protect PANCREATIC ISLETS against damage by CYTOKINES and prevent myocardial and pulmonary REPERFUSION INJURY. (See all compounds classified as Free Radical Scavengers.)
Cholagogues and Choleretics
Gastrointestinal agents that stimulate the flow of bile into the duodenum (cholagogues) or stimulate the production of bile by the liver (choleretic). (See all compounds classified as Cholagogues and Choleretics.)
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
The study described here has investigated the bioavailability of ferulic acid in humans, from tomato consumption, through the monitoring of the pharmacokinetics of excretion in relation to intake. The results show that the peak time for maximal urinary excretion is approximately 7 hr and the recovery of ferulic acid in the urine, on the basis of total free ferulic acid and feruloyl glucuronide excreted, is 11-25% of that ingested.
Bourne LC, Rice Evans C; Biochem Biophys Res Commun 253 (2): 222-7 (1999)
The ... study investigated the urinary excretion of free and conjugated ferulic acid, present in quantitatively detectable amounts in French maritime pine (Pinus maritima) bark extract (PBE), after oral PBE administration to human subjects. Eleven healthy adult subjects (4 women and 7men) consumed either a single dose (200 mg PBE) or two doses of PBE (100 and 200 mg, respectively) within a 48-hr interval. Two days before the oral administration of PBE and during the urine sample collection period volunteers adhered to a diet low in polyphenols. Aliquots of all urine production were collected over 24 hr. Free and conjugated ferulic acid was assessed in urine by HPLC using diode array detection. A close association between the dietary intake of PBE and the urinary excretion of ferulic acid was detected. Moreover, the results indicate that a considerable proportion of ferulic acid is excreted as glucuronide or sulfate after PBE consumption, varying over the range 2 to 20% between individuals. The kinetics of excretion associated with the administration of 100 mg PBE was quite similar to that obtained after 200 mg PBE. A biphasic trend was evident in a number of subjects. All subjects studied here displayed a significant, although variable level of excretion of ferulic acid after supplementation with PBE, Thus, the data provide evidence that at least a part of the phenolic components of PBE are absorbed, metabolized, and eliminated by humans.
Virgili F et al; Free Radic Biol Med 28 (8): 1249-56 (2000) :
The hydroxycinnamates, intermediates in the phenylpropanoid synthetic pathway, are effective in enhancing the resistance of low-density lipoprotein (LDL) to oxidation in the order caffeic acid greater than ferulic acid greater than p-coumaric acid. It is unclear whether the mode of action of ferulic acid as an antioxidant is based on its activities in the aqueous or the lipophilic phase. Partitioning of 14C-labelled ferulic acid into plasma and its components, LDL and the albumin-rich fractions, has been studied under conditions of maximum aqueous solubility. The majority of ferulic acid associates with the albumin-rich fraction of the plasma, although a proportion is also found to partition between the LDL and aqueous phases; however, ferulic acid does not associate with the lipid portion of the LDL particle, suggesting that it exerts its antioxidant properties from the aqueous phase. This is of particular interest since the results demonstrate that ferulic acid is a more effective antioxidant against LDL oxidation than the hydrophilic antioxidant ascorbic acid.
Castelluccio C et al; Biochem J 316 ( Pt 2)691-4 (1996):
The major constituents of artichoke extracts are hydroxycinnamic acids such as chlorogenic acid, dicaffeoylquinic acids caffeic acid and ferulic acid, and flavonoids such as luteolin and apigenin glycosides. ...Several studies have shown the effect on animal models of artichoke extracts ... . . Results showed a plasma maximum concentration of 6.4 (SD 1.8) ng/mL for chlorogenic acid after 1 hr and its disappearance within 2 hr (P< 0.05). Peak plasma concentrations of 19.5 (SD 6.9) ng/ml for total caffeic acid were reached within 1 h, while ferulic acid plasma concentrations showed a biphasic profile with 6.4 (SD1.5) ng/mL and 8.4 (SD4.6) ng/mL within 1 hr and after 8 hr respectively. ...A significant increase of dihydrocaffeic acid and dihydroferulic acid total levels after 8 hr (P<0.05) /was observed/. No circulating plasma levels of luteolin and apigenin were present.
Azzini E et al; Br J Nutr 97 (5): 963-9 (2007):
The bioavailability of ferulic acid (FA; 3-methoxy-4-hydroxycinnamic acid) and its metabolites was investigated in rat plasma and urine after an oral short-term ingestion of 5.15 mg/kg of FA. Free FA, glucuronoconjugates, and sulfoconjugates were quickly detected in plasma with a peak of concentration found 30 min after ingestion. Sulfoconjugates were the main derivates ( approximately 50%). In urine, the cumulative excretion of total metabolites reached a plateau 1.5 h after ingestion, and approximately 40% were excreted by this way. Free FA recovered in urine represented only 4.9 +/-1.5% of the native FA consumed by rats. Glucuronoconjugates and sulfoconjugates represented 0.5 +/- 0.3 and 32.7 +/- 7.3%, respectively. These results suggested that a part of FA incorporated in the diet was quickly absorbed and largely metabolized in sulfoconjugates before excretion in urine.
Rondini F et al; J Agric Food Chem 50 (10): 3037-41(2002):
Ferulic acid (FA) is a phytochemical commonly found in fruits and vegetables such as tomatoes, sweet corn and rice bran. It arises from metabolism of phenylalanine and tyrosine by Shikimate pathway in plants.
PMID:18188410 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2127228 Srinivasan M et al; J Clin Biochem Nutr 40 (2): 92-100 (2007)
Ferulic Acid has known human metabolites that include (2S,3S,4S,5R)-6-[4-[(E)-2-carboxyethenyl]-2-methoxyphenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
54
PharmaCompass offers a list of Ferulic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ferulic Acid manufacturer or Ferulic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ferulic Acid manufacturer or Ferulic Acid supplier.
PharmaCompass also assists you with knowing the Ferulic Acid API Price utilized in the formulation of products. Ferulic Acid API Price is not always fixed or binding as the Ferulic Acid Price is obtained through a variety of data sources. The Ferulic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ferulic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ferulic Acid, including repackagers and relabelers. The FDA regulates Ferulic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ferulic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ferulic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ferulic Acid supplier is an individual or a company that provides Ferulic Acid active pharmaceutical ingredient (API) or Ferulic Acid finished formulations upon request. The Ferulic Acid suppliers may include Ferulic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Ferulic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Ferulic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ferulic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ferulic Acid GMP manufacturer or Ferulic Acid GMP API supplier for your needs.
A Ferulic Acid CoA (Certificate of Analysis) is a formal document that attests to Ferulic Acid's compliance with Ferulic Acid specifications and serves as a tool for batch-level quality control.
Ferulic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Ferulic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ferulic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Ferulic Acid EP), Ferulic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ferulic Acid USP).
