



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
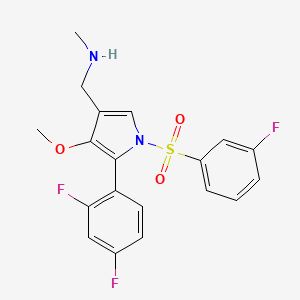

1. 1h-pyrrole-3-methanamine, 5-(2,4-difluorophenyl)-1-((3-fluorophenyl)sulfonyl)-4-methoxy-n-methyl-
2. 5-(2,4-difluorophenyl)-1-((3-fluorophenyl)sulfonyl)-4-methoxy-n-methyl-1h-pyrrole-3-methanamine
3. Dwp-14012
4. Dwp14012
5. Fexuprazan
1. Fexuprazan
2. 1902954-60-2
3. Dwp14012
4. Dwp-14012
5. Be52s2c1qt
6. 1-[5-(2,4-difluorophenyl)-1-(3-fluorobenzene-1-sulfonyl)-4-methoxy-1h-pyrrol-3-yl]-n-methylmethanamine
7. 1h-pyrrole-3-methanamine, 5-(2,4-difluorophenyl)-1-((3-fluorophenyl)sulfonyl)-4-methoxy-n-methyl-
8. 5-(2,4-difluorophenyl)-1-((3-fluorophenyl)sulfonyl)-4-methoxy-n-methyl-1h-pyrrole-3-methanamine
9. Abeprazan [inn]
10. Fexuprazan [inn]
11. Unii-be52s2c1qt
12. Chembl4594445
13. Schembl18196939
14. Glxc-25721
15. Hy-109079
16. Cs-0039254
17. 1-[5-(2,4-difluorophenyl)-1-(3-fluorophenyl)sulfonyl-4-methoxypyrrol-3-yl]-n-methylmethanamine
| Molecular Weight | 410.4 g/mol |
|---|---|
| Molecular Formula | C19H17F3N2O3S |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 410.09119807 g/mol |
| Monoisotopic Mass | 410.09119807 g/mol |
| Topological Polar Surface Area | 68.7 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 618 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)


