
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
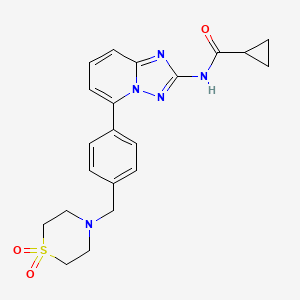

1. 1206101-20-3
2. Glpg0634
3. 1206161-97-8
4. Glpg-0634
5. N-(5-(4-((1,1-dioxidothiomorpholino)methyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanecarboxamide
6. Filgotinib (glpg0634)
7. Filgotinib(glpg0634)
8. Gs-6034 Free Base
9. 3xvl385q0m
10. Gplg0634
11. N-[5-[4-[(1,1-dioxo-1,4-thiazinan-4-yl)methyl]phenyl]-[1,2,4]triazolo[1,5-a]pyridin-2-yl]cyclopropanecarboxamide
12. G146034
13. G-146034
14. N-[5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl][1,2,4]triazolo[1,5-a]pyridin-2-yl]cyclopropanecarboxamide
15. Jyseleca
16. Filgotinib [inn]
17. N-(5-(4-((1,1-dioxidothiomorpholino)methyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanecarboxamide.
18. Filgotinib [usan:inn]
19. Unii-3xvl385q0m
20. Glpg 0634
21. N-(5-{4-[(1,1-dioxidothiomorpholin-4-yl)methyl]phenyl}[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanecarboxamide
22. Glpg0634-analogue
23. Filgotinib (usan/inn)
24. Filgotinib [usan]
25. Filgotinib; Gplg0634
26. Filgotinib [who-dd]
27. Schembl253559
28. Filgotinib Pound Glpg0643)
29. Gtpl7913
30. Chembl3301607
31. Amy3802
32. Dtxsid80152935
33. Ex-a741
34. Bdbm103727
35. Hms3653p15
36. Hms3673e07
37. Bcp08496
38. Mfcd20527867
39. Nsc800100
40. S7605
41. Zinc96174616
42. Akos025291103
43. Ccg-268951
44. Db14845
45. Nsc-800100
46. Sb16799
47. Ncgc00345855-01
48. Ncgc00345855-07
49. As-16295
50. Bf159062
51. Da-33603
52. Da-33604
53. Hy-18300
54. Ft-0700114
55. Ft-0761510
56. Sw220020-1
57. A14232
58. D10871
59. P12798
60. Us8563545, 1
61. A892158
62. J-690063
63. Syn1158;glpg 0634; Glpg-0634; Filgotinib
64. Q19904163
65. Cyclopropanecarboxamide, N-(5-(4-((1,1-dioxido-4-thiomorpholinyl)methyl)phenyl)(1,2,4)triazolo(1,5-a)pyridin-2-yl)-
66. Glpg0634;n-[5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl][1,2,4]triazolo[1,5-a]pyridin-2-yl]cyclopropanecarboxamide
67. N-(5-(4-((1,1-oxo-.lambda.6-thiomorpholin-4-yl)methyl)phenyl((1,2,4)triazolo(1,5-a)pyridin-2-yl)cyclopropanecarboxamide
| Molecular Weight | 425.5 g/mol |
|---|---|
| Molecular Formula | C21H23N5O3S |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 425.15216079 g/mol |
| Monoisotopic Mass | 425.15216079 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 715 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Filgotinib is indicated for the treatment of active moderate to severe rheumatoid arthritis alone or in combination with methotrexate. Filgotinib is currently reserved for patients who are unable to tolerate or who have not responded adequately to one or more disease-modifying anti-rheumatic drugs (DMARDS). Filgotinib is also indicated for treatment of moderately to severely active ulcerative colitis in adult patients who had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic agent.
Treatment of chronic idiopathic arthritis (including rheumatoid arthritis , ankylosing spondylarthritis , psoriatic arthritis , and juvenile idiopathic arthritis )
Rheumatoid arthritis
Jyseleca is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease modifying anti rheumatic drugs (DMARDs). Jyseleca may be used as monotherapy or in combination with methotrexate (MTX).
Ulcerative colitis
Jyseleca is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic agent.
Treatment of Crohn's disease, Treatment of ulcerative colitis
In addition to targeted Janus kinase (JAK) 1 inhibition, filgotinib targets pro-inflammatory cytokine signalling by inhibiting IL-6 induced STAT1 phosphorylation. Serum C-reactive protein levels are also reduced in response to filgotinib administration.
L04AA
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA45 - Filgotinib
Absorption
Filgotinib is rapidly absorbed after oral administration. Median peak plasma concentrations occurred 2-3 hours post-dose for filgotinib and 5 hours post-dose for GS-829845. Steady-state concentrations can be observed in 2-3 days for filgotinib and in 4 days for GS-829845. Food does not appear to have a significant effect on the absorption of filgotinib; therefore, the medication can be administered without regard to food. After repeated oral dosing of filgotinib 200 mg, the reported Cmax and AUC values of filgotinib were 2.15 ug/mL and 6.77 ugxh/mL, respectively. For GS-829845 (the major metabolite) the reported Cmax was 4.43 ug/mL and the reported AUC was 83.2 ugxh/mL.
Route of Elimination
Of the total administered dose of filgotinib, approximately 87% undergoes renal elimination while 15% undergoes faecal elimination.
Carboxylesterase enzymes are involved in the metabolism of filgotinib. The carboxylesterase 2 (CES2) isoform is chiefly responsible for metabolizing filgotinib to its major metabolite, GS-829845. Although carboxylesterase 1 (CES1) plays a less prominent role in the biotransformation of filgotinib, in vitro studies have demonstrated that CES1 will partially compensate in the event of CES2 saturation. GS-829845 is thus far the only major circulating metabolite to have been identified.
The half-life of filgotinib is estimated to be 7 hours, while the half-life of its active metabolite GS-829845 is estimated to be 19 hours.
There are four Janus kinase (JAK) enzymes including JAK1, JAK2, JAK3, and tyrosine kinase 2. JAK1 mediates inflammatory cytokine signaling, while JAK2 and JAK3 are important components of hematologic and immune functions. Filgotinib selectively inhibits JAK1 and is for example nearly 30-fold more selective for JAK1 compared to JAK2. The Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway is implicated in several inflammatory pathologies and has been found to be continuously active in patients who have RA. Sustained activation of this pathway contributes to aberrant processes which lead to disease progression including elevated levels of matrix metalloproteinases (MMPs) and reduced cell apoptosis in RA affected synovial tissues. Filgotinib acts on the JAK-STAT pathway by selectively inhibiting JAK1 phosphorylation and preventing STAT activation, which ultimately results in reduced proinflammatory cytokine signaling.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.

Registrant Name : Korea Ezai Co., Ltd.
Registration Date : 2022-04-01
Registration Number : Su205-27-ND
Manufacturer Name : Gilead Alberta ULC
Manufacturer Address : 1021 Hayter Road, Edmonton, Alberta, T6S 1A1, Canada


NDC Package Code : 59116-7110
Start Marketing Date : 2021-12-16
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Korea Ezai Co., Ltd.
Registration Date : 2022-04-01
Registration Number : Su205-26-ND
Manufacturer Name : Cambrex Charles City, Inc
Manufacturer Address : 1205 11th Street - Charles City, IA 50616, USA



NDC Package Code : 59116-5440
Start Marketing Date : 2020-04-01
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Registrant Name : Korea Ezai Co., Ltd.
Registration Date : 2022-04-01
Registration Number : Su205-26-ND
Manufacturer Name : Cambrex Charles City, Inc
Manufacturer Address : 1205 11th Street - Charles City, IA 50616, USA

Registrant Name : Korea Ezai Co., Ltd.
Registration Date : 2022-04-01
Registration Number : Su205-27-ND
Manufacturer Name : Gilead Alberta ULC
Manufacturer Address : 1021 Hayter Road, Edmonton, Alberta, T6S 1A1, Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 59116-7110
Start Marketing Date : 2021-12-16
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 59116-5440
Start Marketing Date : 2020-04-01
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
About the Company : Beijing Sjar Technology Development Co., Ltd. founded in 2014, it is a high-tech enterprise which specialized in the research and development of active pharmaceutical ingredients a...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

ABOUT THIS PAGE
33
PharmaCompass offers a list of Filgotinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Filgotinib manufacturer or Filgotinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Filgotinib manufacturer or Filgotinib supplier.
PharmaCompass also assists you with knowing the Filgotinib API Price utilized in the formulation of products. Filgotinib API Price is not always fixed or binding as the Filgotinib Price is obtained through a variety of data sources. The Filgotinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Filgotinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Filgotinib, including repackagers and relabelers. The FDA regulates Filgotinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Filgotinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Filgotinib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Filgotinib supplier is an individual or a company that provides Filgotinib active pharmaceutical ingredient (API) or Filgotinib finished formulations upon request. The Filgotinib suppliers may include Filgotinib API manufacturers, exporters, distributors and traders.
click here to find a list of Filgotinib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Filgotinib Drug Master File in Korea (Filgotinib KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Filgotinib. The MFDS reviews the Filgotinib KDMF as part of the drug registration process and uses the information provided in the Filgotinib KDMF to evaluate the safety and efficacy of the drug.
After submitting a Filgotinib KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Filgotinib API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Filgotinib suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Filgotinib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Filgotinib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Filgotinib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Filgotinib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Filgotinib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Filgotinib suppliers with NDC on PharmaCompass.
Filgotinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Filgotinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Filgotinib GMP manufacturer or Filgotinib GMP API supplier for your needs.
A Filgotinib CoA (Certificate of Analysis) is a formal document that attests to Filgotinib's compliance with Filgotinib specifications and serves as a tool for batch-level quality control.
Filgotinib CoA mostly includes findings from lab analyses of a specific batch. For each Filgotinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Filgotinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Filgotinib EP), Filgotinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Filgotinib USP).

