
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
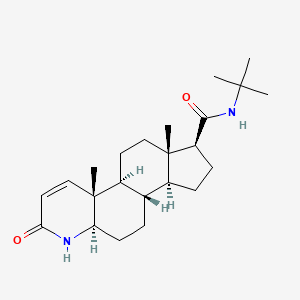

1. Chibro Proscar
2. Chibro-proscar
3. Eucoprost
4. Mk 906
5. Mk-906
6. Mk906
7. Propecia
8. Propeshia
9. Proscar
1. 98319-26-7
2. Proscar
3. Propecia
4. Finastid
5. Prostide
6. Chibro-proscar
7. Mk-906
8. Finpecia
9. Finasterida
10. Finasteridum
11. Mk 906
12. (5alpha,17beta)-(1,1-dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide
13. (4ar,4bs,6as,7s,9as,9bs,11ar)-n-tert-butyl-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxamide
14. Chembl710
15. Nsc-741485
16. N-tert-butyl-3-oxo-4-aza-5alpha-androst-1-ene-17beta-carboxamide
17. 57gno57u7g
18. Chebi:5062
19. (1s,3as,3bs,5ar,9ar,9bs,11as)-n-tert-butyl-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide
20. (4ar,4bs,6as,7s,9as,9bs,11ar)-n-(tert-butyl)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxamide
21. Nsc-759318
22. Finasteridum [inn-latin]
23. Finasterida [inn-spanish]
24. Dsstox_cid_625
25. Dsstox_rid_75699
26. Dsstox_gsid_20625
27. (4ar,4bs,6as,7s,9as,9bs,11ar)-n-(1,1-dimethylethyl)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxamide
28. N-tert-butyl-3-oxo-4-aza-5alpha-androst-1-en-17beta-carboxamide
29. Andozac
30. 4-azaandrost-1-ene-17-carboxamide, N-(1,1-dimethylethyl)-3-oxo-, (5alpha,17beta)-
31. Propecia (tn)
32. Smr000466304
33. Proscar (tn)
34. Mk 0906
35. Mk-0906
36. Ccris 7438
37. Hsdb 6793
38. Mk906
39. Sr-01000759414
40. Brn 4269024
41. Unii-57gno57u7g
42. L-652,931
43. 17beta-(n-tert-butylcarbamoyl)-4-aza-5 Alpha-androst-1-en-3-one
44. Ncgc00016965-01
45. Finasteride [usan:usp:inn:ban]
46. N-(2-methyl-2-propyl)-3-oxo-4-aza-5-alpha-androst-1-ene-17-beta-carboxamide
47. Cas-98319-26-7
48. Mfcd00869737
49. Ym-152
50. Ks-1058
51. Finasteride (proscar)
52. Finasteride [mi]
53. Prestwick0_000717
54. Prestwick1_000717
55. Prestwick2_000717
56. Prestwick3_000717
57. Finasteride [inn]
58. Finasteride [jan]
59. Finasteride [usan]
60. Finasteride [vandf]
61. Schembl5509
62. Finasteride [mart.]
63. Bspbio_000933
64. Finasteride [who-dd]
65. Mls000759404
66. Mls001165768
67. Mls001424046
68. Finasteride (jan/usp/inn)
69. Spbio_002854
70. Bpbio1_001027
71. Gtpl6818
72. Dtxsid3020625
73. N-tert-butyl-3-oxo-4-aza-5.alpha.-androst-1-ene-17.beta.-carboxamide
74. Finasteride [ep Impurity]
75. Finasteride [orange Book]
76. 4-azaandrost-1-ene-17-carboxamide, N-(1,1-dimethylethyl)-3-oxo-, (5.alpha.,17.beta.)-
77. Bcpp000229
78. Finasteride [ep Monograph]
79. Finasteride [usp Impurity]
80. Hms1570o15
81. Hms2051f09
82. Hms2090g22
83. Hms2097o15
84. Hms2235l23
85. Hms3714o15
86. Finasteride [usp Monograph]
87. 4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide
88. Act02599
89. Entadfi Component Finasteride
90. Ex-a1951
91. Zinc3782599
92. Tox21_110717
93. Tox21_201506
94. Tox21_302744
95. Bdbm50334788
96. Nsc741485
97. Nsc757443
98. S1197
99. Akos015894916
100. Tox21_110717_1
101. Bcp9000685
102. Ccg-100937
103. Cs-1767
104. Db01216
105. Finasteride Component Of Entadfi
106. Finasteride, >=98% (hplc), Powder
107. Nc00187
108. Nsc 741485
109. Nsc 759318
110. Nsc-757443
111. Ncgc00093560-05
112. Ncgc00256334-01
113. Ncgc00259057-01
114. 140375-21-9
115. Bf164456
116. Cpd000466304
117. Hy-13635
118. Bcp0726000222
119. Ab00513901
120. D00321
121. Ab00513901-07
122. Ab00513901-08
123. Ab00513901_09
124. Finasteride, Vetranal(tm), Analytical Standard
125. 319f267
126. A845840
127. Q424167
128. Sr-01000759414-4
129. Sr-01000759414-6
130. Brd-k01095011-001-03-5
131. Brd-k01095011-001-15-9
132. Finasteride, British Pharmacopoeia (bp) Reference Standard
133. Finasteride, European Pharmacopoeia (ep) Reference Standard
134. ([1-phenyl-meth-(e)-ylidene]-amino)-aceticacidethylester
135. (17beta-(n-tert-butylcarbamoyl)-4-aza-5alpha-androst-1-en-3-one
136. Finasteride, United States Pharmacopeia (usp) Reference Standard
137. (5?,17?)-n-(1,1-dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide
138. 4-azaandrost-1-ene-17-carboxamide,1-dimethylethyl)-3-oxo-, (5.alpha., 17.beta.)-
139. Finasteride For Peak Identification, European Pharmacopoeia (ep) Reference Standard
140. (1s,2r,7r,10s,11s,14s,15s)-n-tert-butyl-2,15-dimethyl-5-oxo-6-azatetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-3-ene-14-carboxamide
141. (1s,3as,3bs,5ar,9ar,9bs,11as)-n-isobutyl-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide
142. (1s,3as,3bs,5ar,9ar,9bs,11as)-n-isobutyl-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide;finasteride
143. (4ar,4bs,6as,7s,9as,9bs,11ar)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide
144. (4ar,4bs,6as,9as,9bs,11ar)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide
145. (4ar,6as)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide
146. (4ar,6as,11ar)-4a,6a-dimethyl-2-oxo-hexadecahydro-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide
147. (4ar,6as,7s)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide
148. (4ar,6as,7s,11ar)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide
149. (r)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide
150. 1h-indeno[5,4-f]quinoline-7-carboxamide, N-(1,1-dimethylethyl)-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-4a,6a-dimethyl-2-oxo-, (4ar,4bs,6as,7s,9as,9bs,11ar)-
151. 4a,6a,9a-trimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid (finasteride)
152. 4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1h-indeno[5,4-f]quinoline-7-carboxylic Acid Tert-butylamide(finasteride)
| Molecular Weight | 372.5 g/mol |
|---|---|
| Molecular Formula | C23H36N2O2 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 372.277678395 g/mol |
| Monoisotopic Mass | 372.277678395 g/mol |
| Topological Polar Surface Area | 58.2 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 678 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Finasteride |
| PubMed Health | Finasteride (By mouth) |
| Drug Classes | Alopecia Agent, Benign Prostatic Hypertrophy Agent |
| Drug Label | Finasteride, USP, a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5-reductase, an intracellular enzyme that converts the androgen testosterone into 5-dihydrotestosterone (DHT).Finasteride is 4-Azaandrost-1-ene-17-car... |
| Active Ingredient | Finasteride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 1mg |
| Market Status | Tentative Approval; Prescription |
| Company | Watson Labs; Mylan Pharms; Actavis Elizabeth; Teva; Accord Hlthcare; Hetero Labs Ltd Iii; Aurobindo Pharma; Zydus Pharms Usa; Dr Reddys Labs; Gedeon Richter Usa; Sun Pharma Global; Mylan |
| 2 of 6 | |
|---|---|
| Drug Name | Propecia |
| PubMed Health | Finasteride (By mouth) |
| Drug Classes | Alopecia Agent, Benign Prostatic Hypertrophy Agent |
| Drug Label | PROPECIA (finasteride) tablets contain finasteride as the active ingredient. Finasteride, a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5-reductase, an intracellular enzyme that converts the androgen testosterone int... |
| Active Ingredient | Finasteride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg |
| Market Status | Prescription |
| Company | Merck |
| 3 of 6 | |
|---|---|
| Drug Name | Proscar |
| Drug Label | PROSCAR (finasteride), a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5-reductase, an intracellular enzyme that converts the androgen testosterone into 5-dihydrotestosterone (DHT).Finasteride is 4-azaandrost-1-ene-1... |
| Active Ingredient | Finasteride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg |
| Market Status | Prescription |
| Company | Merck |
| 4 of 6 | |
|---|---|
| Drug Name | Finasteride |
| PubMed Health | Finasteride (By mouth) |
| Drug Classes | Alopecia Agent, Benign Prostatic Hypertrophy Agent |
| Drug Label | Finasteride, USP, a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5-reductase, an intracellular enzyme that converts the androgen testosterone into 5-dihydrotestosterone (DHT).Finasteride is 4-Azaandrost-1-ene-17-car... |
| Active Ingredient | Finasteride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 1mg |
| Market Status | Tentative Approval; Prescription |
| Company | Watson Labs; Mylan Pharms; Actavis Elizabeth; Teva; Accord Hlthcare; Hetero Labs Ltd Iii; Aurobindo Pharma; Zydus Pharms Usa; Dr Reddys Labs; Gedeon Richter Usa; Sun Pharma Global; Mylan |
| 5 of 6 | |
|---|---|
| Drug Name | Propecia |
| PubMed Health | Finasteride (By mouth) |
| Drug Classes | Alopecia Agent, Benign Prostatic Hypertrophy Agent |
| Drug Label | PROPECIA (finasteride) tablets contain finasteride as the active ingredient. Finasteride, a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5-reductase, an intracellular enzyme that converts the androgen testosterone int... |
| Active Ingredient | Finasteride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg |
| Market Status | Prescription |
| Company | Merck |
| 6 of 6 | |
|---|---|
| Drug Name | Proscar |
| Drug Label | PROSCAR (finasteride), a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5-reductase, an intracellular enzyme that converts the androgen testosterone into 5-dihydrotestosterone (DHT).Finasteride is 4-azaandrost-1-ene-1... |
| Active Ingredient | Finasteride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg |
| Market Status | Prescription |
| Company | Merck |
Enzyme Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Treatment of benign prostatic hypertrophy
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1250
Antiandrogen therapy appears to produce a 30 to 40% decrease in the volume of the hyperplastic prostate after 3 to 6 months of therapy. Longer treatment may result in further prostatic regression, although this remains to be seen. Biopsy studies suggest that epithelial regression occurs to a much more significant degree than does stromal regression, but this finding may simply reflect the relatively longer turnover of the stromal cell population. The significant placebo effect of oral medication in patients with benign prostatic hyperplasia makes interpretation of clinical symptomatology and uro-flow data difficult. Analysis of symptom improvement is further complicated by the relatively slow improvement of patients on antiandrogen therapy, in contrast to surgery, in which relief is immediate. In addition to limited stromal involution and inadequate treatment duration, other biologic factors may limit the clinical efficacy of antiandrogen therapy. Most importantly, prostatic involution may not necessarily decrease urethral resistance. In addition, obstruction induced detrusor dysfunction may persist after relief of outflow obstruction in some patients, as it does after surgery. Incomplete antiandrogen action of the compounds, as well as compliance issues, may likewise limit efficacy. Although there are no data to suggest that the 5 alpha-reductase inhibitor finasteride will be more effective than other antiandrogen compounds in the treatment of benign prostatic hyperplasia, preliminary studies suggest that it has less toxicity. If long-term studies validate a modest but significant clinical response rate and preservation of sexual function, then finasteride therapy may well be acceptable to a subgroup of men presenting with the symptoms of benign prostatic hyperplasia.
PMID:1695786 McConnell JD; Urol Clin North Am 17 (3): 661-70 (1990)
Finasteride is indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to improve symptoms, reduce the risk of acute urinary retention, and reduce the risk of the need for surgery including transurethral resection of the prostate (TURP) and prostatectomy. A combination product with [tadalafil] is also used for the symptomatic treatment of BPH for up to 26 weeks. Finasteride is also indicated for the treatment of male pattern hair loss (androgenetic alopecia, hereditary alopecia, or common male baldness) in male patients.
Treatment of androgenetic alopecia
Finasteride is an antiandrogenic compound that works by suppressing the production of serum and intraprostatic dihydrotestosterone (DHT) in men via inhibiting the enzyme responsible for the biosynthesis of DHT. The maximum effect of a rapid reduction in serum DHT concentration is expected to be observed 8 hours following administration of the first dose. In a single man receiving a single oral dose of 5 mg finasteride for up to 4 years, there was a reduction in the serum DHT concentrations by approximately 70% and the median circulating level of testosterone increased by approximately 10-20% within the physiologic range. In a double-blind, placebo-controlled study, finasteride reduced intraprostatic DHT level by 91.4% but finasteride is not expected to decrease the DHT levels to castrate levels since circulating testosterone is also converted to DHT by the type 1 isoenzyme expressed in other tissues. It is expected that DHT levels return to normal within 14 days upon discontinuation of the drug. In a study of male patients with benign prostatic hyperplasia prior to prostatectomy, the treatment with finasteride resulted in an approximate 80% lower DHT content was measured in prostatic tissue removed at surgery compared to placebo. While finasteride reduces the size of the prostate gland by 20%, this may not correlate well with improvement in symptoms. The effects of finasteride are reported to be more pronounced in male patients with enlarged prostates (>25 mL) who are at the greatest risk of disease progression. In phase III clinical studies, oral administration of finasteride in male patients with male pattern hair loss promoted hair growth and prevented further hair loss by 66% and 83% of the subjects, respectively, which lasted during two years' treatment. The incidences of these effects in treatment groups were significantly higher than that of the group receiving a placebo. Following finasteride administration, the levels of DHT in the scalp skin was shown to be reduced by more than 60%, indicating that the DHT found in scalp is derived from both local DHT production and circulating DHT. The effect of finasteride on scalp DHT is likely seen because of its effect on both local follicular DHT levels as well as serum DHT levels.. There is evidence from early clinical observations and controlled studies that finasteride may reduce bleeding of prostatic origin.
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
5-alpha Reductase Inhibitors
Drugs that inhibit 3-OXO-5-ALPHA-STEROID 4-DEHYDROGENASE. They are commonly used to reduce the production of DIHYDROTESTOSTERONE. (See all compounds classified as 5-alpha Reductase Inhibitors.)
G04CB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AX - Other dermatologicals
D11AX10 - Finasteride
G - Genito urinary system and sex hormones
G04 - Urologicals
G04C - Drugs used in benign prostatic hypertrophy
G04CB - Testosterone-5-alpha reductase inhibitors
G04CB01 - Finasteride
Absorption
Finasteride is well absorbed following oral administration and displays a slow accumulation phase after multiple dosing.[lablel] In healthy male subjects receiving oral finasteride, the mean oral bioavailability was 65% for 1 mg finasteride and 63% for 5 mg finasteride, and the values ranged from 26 to 170% for 1 mg dose and from 34 to 108% for 5 mg dose, respectively. It is reported that food intake does not affect the oral bioavailability of the drug. The peak plasma concentrations (Cmax) averaged 37 ng/mL (range, 27-49 ng/mL) and was reached 1-2 hours post administration. The AUC(0-24 hr) was 53 ngxhr/mL (range, 20-154 ngxhr/mL). The plasma concentrations and AUC are reported to be higher in elderly male patients aged 70 years or older.
Route of Elimination
In healthy subjects, about 32-46% of total oral dose of finasteride was excreted in the urine in the form of metabolites while about 51-64% of the dose was excreted in the feces. In patients with renal impairment, the extent of urinary excretion of finasteride is expected to be decreased while the fecal excretion is increased.
Volume of Distribution
The volume of distribution is 76 L at steady state, ranging from 44 to 96 L. Finasteride has been shown to cross the blood brain barrier but does not appear to distribute preferentially to the CSF. It is not known whether finasteride is excreted in human milk.
Clearance
In healthy young subjects (n=15), the mean plasma clearance of finasteride was 165 mL/min with the range between 70 and 279 mL/min.
Finasteride undergoes extensive hepatic metabolism predominantly mediated by the cytochrome P450 3A4 (CYP3A4) enzyme to form the t-butyl side chain monohydroxylated and monocarboxylic acid metabolites. Theses metabolites retain less than 20% of the pharmacological activity of the parent compound.
Finasteride has known human metabolites that include N-(1-Hydroxy-2-methylpropan-2-yl)-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
In healthy young subjects receiving finasteride, the mean elimination half-life in plasma was 6 hours ranging from 3 to 16 hours. In elderly patients over the age of 70 years, the half-life is prolonged to 8 hours.
Finasteride acts as a competitive and specific inhibitor of Type II 5-reductase, a nuclear-bound steroid intracellular enzyme primarily located in the prostatic stromal cell that converts the androgen testosterone into the more active metabolite, 5-dihydrotestosterone (DHT). DHT is considered to be the primary androgen playing a role in the development and enlargement of the prostate gland. It serves as the hormonal mediator for the hyperplasia upon accumulation within the prostate gland. DHT displays a higher affinity towards androgen receptors in the prostate gland compared to testosterone and by acting on the androgen receptors, DHT modulates genes that are responsible for cell proliferation. Responsible for the production of DHT together with type I 5-reductase, the type II 5-reductase isozyme is primarily found in the prostate, seminal vesicles, epididymides, and hair follicles as well as liver. Although finasteride is 100-fold more selective for type II 5-reductase than for the type I isoenzyme, chronic treatment with this drug may have some effect on type I 5-reductase, which is predominantly expressed in sebaceous glands of most regions of skin, including the scalp, and liver. It is proposed that the type I 5-reductase and type II 5-reductase is responsible for the production of one-third and two-thirds of circulating DHT, respectively. The mechanism of action of Finasteride is based on its preferential inhibition of Type II 5-reductase through the formation of a stable complex with the enzyme _in vitro_ and _in vivo_. Finasteride works selectively, where it preferentially displays a 100-fold selectivity for the human Type II 5-reductase over type I enzyme. Inhibition of Type II 5-reductase blocks the peripheral conversion of testosterone to DHT, resulting in significant decreases in serum and tissue DHT concentrations, minimal to moderate increase in serum testosterone concentrations, and substantial increases in prostatic testosterone concentrations. As DHT appears to be the principal androgen responsible for stimulation of prostatic growth, a decrease in DHT concentrations will result in a decrease in prostatic volume (approximately 20-30% after 6-24 months of continued therapy). It is suggested that increased levels of DHT can lead to potentiated transcription of prostaglandin D2, which promotes the proliferation of prostate cancer cells. In men with androgenic alopecia, the mechanism of action has not been fully determined, but finasteride has shown to decrease scalp DHT concentration to the levels found in the hairy scalp, reduce serum DHT, increase hair regrowth, and slow hair loss. Another study suggests that finasteride may work to reduce bleeding of prostatic origin by inhibiting vascular endothelial growth factor (VEGF) in the prostate, leading to atrophy and programmed cell death. This may bestow the drug therapeutic benefits in patients idiopathic prostatic bleeding, bleeding during anticoagulation, or bleeding after instrumentation.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
77
PharmaCompass offers a list of Finasteride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Finasteride manufacturer or Finasteride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Finasteride manufacturer or Finasteride supplier.
PharmaCompass also assists you with knowing the Finasteride API Price utilized in the formulation of products. Finasteride API Price is not always fixed or binding as the Finasteride Price is obtained through a variety of data sources. The Finasteride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Finasteride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Finasteride, including repackagers and relabelers. The FDA regulates Finasteride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Finasteride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Finasteride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Finasteride supplier is an individual or a company that provides Finasteride active pharmaceutical ingredient (API) or Finasteride finished formulations upon request. The Finasteride suppliers may include Finasteride API manufacturers, exporters, distributors and traders.
click here to find a list of Finasteride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Finasteride DMF (Drug Master File) is a document detailing the whole manufacturing process of Finasteride active pharmaceutical ingredient (API) in detail. Different forms of Finasteride DMFs exist exist since differing nations have different regulations, such as Finasteride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Finasteride DMF submitted to regulatory agencies in the US is known as a USDMF. Finasteride USDMF includes data on Finasteride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Finasteride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Finasteride suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Finasteride Drug Master File in Japan (Finasteride JDMF) empowers Finasteride API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Finasteride JDMF during the approval evaluation for pharmaceutical products. At the time of Finasteride JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Finasteride suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Finasteride Drug Master File in Korea (Finasteride KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Finasteride. The MFDS reviews the Finasteride KDMF as part of the drug registration process and uses the information provided in the Finasteride KDMF to evaluate the safety and efficacy of the drug.
After submitting a Finasteride KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Finasteride API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Finasteride suppliers with KDMF on PharmaCompass.
A Finasteride CEP of the European Pharmacopoeia monograph is often referred to as a Finasteride Certificate of Suitability (COS). The purpose of a Finasteride CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Finasteride EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Finasteride to their clients by showing that a Finasteride CEP has been issued for it. The manufacturer submits a Finasteride CEP (COS) as part of the market authorization procedure, and it takes on the role of a Finasteride CEP holder for the record. Additionally, the data presented in the Finasteride CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Finasteride DMF.
A Finasteride CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Finasteride CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Finasteride suppliers with CEP (COS) on PharmaCompass.
A Finasteride written confirmation (Finasteride WC) is an official document issued by a regulatory agency to a Finasteride manufacturer, verifying that the manufacturing facility of a Finasteride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Finasteride APIs or Finasteride finished pharmaceutical products to another nation, regulatory agencies frequently require a Finasteride WC (written confirmation) as part of the regulatory process.
click here to find a list of Finasteride suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Finasteride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Finasteride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Finasteride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Finasteride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Finasteride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Finasteride suppliers with NDC on PharmaCompass.
Finasteride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Finasteride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Finasteride GMP manufacturer or Finasteride GMP API supplier for your needs.
A Finasteride CoA (Certificate of Analysis) is a formal document that attests to Finasteride's compliance with Finasteride specifications and serves as a tool for batch-level quality control.
Finasteride CoA mostly includes findings from lab analyses of a specific batch. For each Finasteride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Finasteride may be tested according to a variety of international standards, such as European Pharmacopoeia (Finasteride EP), Finasteride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Finasteride USP).
