
Synopsis
Synopsis
0
USDMF
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
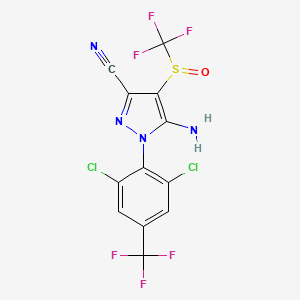

1. 5-amino-1-(2,6-dichloro-alpha,alpha,alpha-trifluoro-p-tolyl)-4-trifluoromethylsulfinylpyrazole-3-carbonitile
1. 120068-37-3
2. Termidor
3. Fipronil [iso]
4. Rm 1601
5. Mb 46030
6. 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-(trifluoromethylsulfinyl)pyrazole-3-carbonitrile
7. Nsc-758960
8. Chembl101326
9. 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1h-pyrazole-3-carbonitrile
10. 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(trifluoromethyl)sulfinyl]-1h-pyrazole-3-carbonitrile
11. Chebi:83394
12. Fipronil (ema Epar: Veterinary)
13. Qgh063955f
14. Fipronil (jan)
15. Rm-1601
16. Ncgc00094574-06
17. Mb-46030
18. Fipronil [jan]
19. 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylsulfinylpyrazole
20. Dsstox_cid_14609
21. Dsstox_rid_79176
22. Dsstox_gsid_34609
23. Fluocyanobenpyrazole
24. (rs)-5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-(trifluoromethylsulfinyl)pyrazole-3-carbonitrile
25. 1h-pyrazole-3-carbonitrile, 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-
26. 1h-pyrazole-3-carbonitrile, 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(trifluoromethyl)sulfinyl]-
27. 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-(trifluoromethane)sulfinyl-1h-pyrazole-3-carbonitrile
28. Cas-120068-37-3
29. (+/-)-fipronil
30. Hsdb 7051
31. Unii-qgh063955f
32. M & B 46030
33. Frontline
34. Fipronil [inn:ban]
35. Fipronil [hsdb]
36. Fipronil [mi]
37. Fipronil [mart.]
38. Upcmld-dp011
39. Ec 424-610-5
40. Cbiol_001754
41. Schembl15088
42. Bspbio_001315
43. Bspbio_002266
44. Kbiogr_000035
45. Kbioss_000035
46. Mls004712079
47. Spectrum1505354
48. Dtxsid4034609
49. Upcmld-dp011:001
50. Upcmld-dp011:002
51. Bcbcmap01_000152
52. Kbio2_000035
53. Kbio2_002603
54. Kbio2_005171
55. Kbio3_000069
56. Kbio3_000070
57. Bio1_000040
58. Bio1_000529
59. Bio1_001018
60. Bio2_000035
61. Bio2_000515
62. Hms1361b17
63. Hms1791b17
64. Hms1922f08
65. Hms1989b17
66. Hms2093d09
67. Hms3402b17
68. Pharmakon1600-01505354
69. Broadline Component Fipronil
70. 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(s)-trifluoromethylsulfinyl]pyrazole-3-carbonitrile
71. Bcp14931
72. Hy-b0822
73. Tox21_111300
74. Tox21_301114
75. Ac-427
76. Bdbm50106148
77. Cm-102
78. Fipronil 100 Microg/ml In Methanol
79. Mfcd00867812
80. Nsc758960
81. S5366
82. Fipronil 1000 Microg/ml In Acetone
83. Akos015960931
84. Fipronil Component Of Broadline
85. Fluocyanobenpyrazole, Taurus, Termidor
86. Tox21_111300_1
87. Ccg-213975
88. Ks-5066
89. Nsc 758960
90. Fipronil 100 Microg/ml In Acetonitrile
91. Idi1_033785
92. Ncgc00094574-01
93. Ncgc00094574-02
94. Ncgc00094574-03
95. Ncgc00094574-05
96. Ncgc00094574-07
97. Ncgc00094574-08
98. Ncgc00094574-09
99. Ncgc00094574-10
100. Ncgc00094574-11
101. Ncgc00094574-12
102. Ncgc00094574-13
103. Ncgc00094574-15
104. Ncgc00255014-01
105. Smr000777967
106. Sbi-0206839.p001
107. Db-041522
108. Cs-0012838
109. F0822
110. Fipronil, Pestanal(r), Analytical Standard
111. Ft-0631097
112. D01042
113. Ab00643326_02
114. 068f373
115. A934386
116. Q415933
117. Sr-05000002006
118. Q-201115
119. Sr-05000002006-1
120. Brd-a50675702-001-03-0
121. Brd-a50675702-001-04-8
122. Fipronil, Certified Reference Material, Tracecert(r)
123. 5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyano-4-trifluoromethylsulfinylpyrazole
124. 5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyano-4-trifluoromethylsulphinylpyrazole
125. 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylsulfinyl-pyrazole
126. 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylsulfinylpyrazol E
127. 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylsulphinylpyrazole
128. (rs)-5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-(trifluoromethylsulfinyl)pyrazole-3-carbonitrile
129. 5-amino-1 -(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylsulfinyl-1 H-pyrazole-3-carbonitrile
130. 5-amino-1-(2,6-dichloro-.alpha.,.alpha.,.alpha.-trifluoro-p-tolyl)-4-((trifluoromethyl)sulfinyl)pyrazole-3-carbonitrile
131. 5-amino-1-(2,6-dichloro-4-(trifluoromethyl) Phenyl)-4-((trifluoromethyl) Sulfinyl)-1h-pyrazol-3-carbonitrile
132. 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylsulfinyl)-1h-pyrazole-3-carbonitrile
133. 5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyano-4-trifluoromethylsulphinyl Pyrazole
134. 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-3-cyano-4-(trifluoromethyl)sulfinylpyrazole
135. 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-(trifluoromethyl)sulfinylpyrazole-3-carbonitrile
136. 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-trifluoromethanesulfinyl-1h-pyrazole-3-carbonitrile
137. 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl) -4-trifluoromethylsulphinylpyrazole
138. Fipronil 10 Microg/ml In Acetonitrile. Short Expiry Date Due To Chemical Nature Of Component(s)
| Molecular Weight | 437.1 g/mol |
|---|---|
| Molecular Formula | C12H4Cl2F6N4OS |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 2 |
| Exact Mass | 435.9387063 g/mol |
| Monoisotopic Mass | 435.9387063 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 599 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
VET: Ectoparasiticide.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 748
VET: Fipronil is a broad-spectrum pesticide with activity against fleas, ticks, mites, and lice.
Kahn, C.M (ed.).; The Merck Veterinary Manual 10th Edition. Merck & Co. Whitehouse Station NJ. 2010, p. 2361
Antiparasitic Agents
Drugs used to treat or prevent parasitic infections. (See all compounds classified as Antiparasitic Agents.)
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
... In this study, the tissue distribution, the metabolic fate, and the elimination of fipronil was investigated in rats using radiolabeled fipronil. When a single oral dose of (14)C-fipronil (10 mg/kg b.w.) was given to rats, the proportion of dose eliminated in urine and feces 72 hr after dosing was ca 4% for each route. At the end of the experiment the highest levels of radioactivity were found in adipose tissue and adrenals. The main part of the radioactivity present in investigated tissues (adipose tissue, adrenals, liver, kidney, testes) was due to fipronil-sulfone. Five additional metabolites, isolated from urine were characterized by LC-MS/MS. Most of them are formed by the loss of the trifluoromethylsulphinyl group and subsequent hydroxylation and/or conjugation to glucuronic acid or sulfate. In conclusion, the retention of the metabolite fipronil sulfone in tissues following fipronil administration raises the question of the potential toxicity of this insecticide.
PMID:24016625 Cravedi JP et al; Chemosphere 93 (10): 2276-83 (2013)
To investigate the localization of fipronil in dog skin, (14)C-fipronil was topically applied to a male beagle dog (spot-on administration) at the therapeutic dose of 10 mg/kg. By means of autohistoradiography, the radioactivity was precisely detected in the skin and appendages at various intervals after application. Radioactivity was predominantly observed within the stratum corneum, the viable epidermis, and in the pilo-sebaceous units (mainly in the sebaceous glands and epithelial layers). (14)C-fipronil was significantly detected in these structures up to 56 days post-treatment, in the application zone (neck) but also in the lumbar zone, thus indicating the mechanical displacement of fipronil. No radioactivity was detected in either the dermal or the hypodermal layers, confirming the low percutaneous passage of fipronil.
PMID:9358201 Cochet P et al; Eur J Drug Metab Pharmacokinet 22 (3): 211-6 (1997)
Absorption of (14)C-fipronil through epidermal membranes of humans, rabbits, and rats was measured in vitro in horizontal glass diffusion cells. ... The epidermal membranes were set up as a barrier between the two halves of the diffusion cells, and the absorption rates of a neat suspension of fipronil (200 g/L) as a formulation in EP60145A (a formulation base) and of two aqueous dilutions of the formulation containing 0.2 and 4 g/L of fipronil suspended in EP 60145A were determined ... . Fipronil at doses of 4 and 200 g/L penetrated rabbit and rat epidermal membranes to a greater extent than those of humans, whereas at 0.2 g/L the extent of penetration was similar through human and rat skin. The extent of penetration increased with time across species. The % of the applied dose that had penetrated the different membranes after 8 hr was 0.08% through rat epidermal membranes, 0.07% through rabbit membranes, and 0.01% through human membranes for the neat formulation; 0.14, 0.67, and 0.07% of the dose of 4.0 g/L active ingredient; and 0.9, 13.9, and 0.9% of the dose of 0.2 g/L active ingredient, respectively. At the dose of 4.0 g/L, fipronil penetrated the skin of all three species more slowly than either testosterone or hydrocortisone. These two reference permeants were selected because their intrinsic rates of dermal penetration differ by two orders of magnitude, that of testosterone being faster. On the basis of the results for these two compounds, fipronil was considered to be a slow penetrant when applied as a formulation in EP 60145A.
FAO/WHO; Pesticide Residues in Food-Fipronil (1997). Available from, as of June 18, 2018: https://www.inchem.org/documents/jmpr/jmpmono/v097pr09.htm
In a study of the absorption, distribution, metabolism, and excretion of fipronil in ruminants, [phenyl(U)-14C]-fipronil (19.2 mCi/mmol) was administered orally by capsule twice daily before feeding to three lactating goats at a dose of 0.05, 2, or 10 ppm for 7 days; assuming a daily intake of 2.0 kg dry matter, these doses are approx equivalent to nominal daily doses of 0.1, 4, and 20 mg, respectively. Milk was collected twice daily. The animals were killed about 24 hr after admin of the final dose and tissues obtained for analysis. The recovery of radiolabel in urine, milk, and tissues indicated that the min absorption of test material was about 19% at 0.05 ppm, 33% at 2 ppm, and 15% at 10 ppm. Of the administered radiolabel, 18-64% was recovered in feces, 1-5% in the milk, and 8-25% in the tissues. Total recovery was similar at the low (83%) and high doses (77%) but was somewhat lower at the intermediate dose (50%). The greatest contributor to the difference in recovery between the animals at the low and high doses and those at the intermediate dose was the amount of radiolabel excreted in the feces: 18% of the total radiolabel administered at 2 ppm, 64% at 0.05 ppm, and 61% at 10 ppm. The reason for this difference is not clear. The greatest total tissue residues were observed in omental and renal fat (about 1.9 ppm at the 10 ppm dose), followed by liver (0.86 ppm) and much lower concns in kidney, milk (0.17 ppm), and skeletal muscle.
FAO/WHO; Pesticide Residues in Food-Fipronil (1997). Available from, as of June 18, 2018: https://www.inchem.org/documents/jmpr/jmpmono/v097pr09.htm
For more Absorption, Distribution and Excretion (Complete) data for Fipronil (15 total), please visit the HSDB record page.
Fipronil gives effective control of early shoot borer and termites in sugarcane. The persistence and metabolism of fipronil in sugarcane leaves and juice were studied following application of fipronil (Regent 0.3 G) at 75 and 300 g a.i./ha. Samples of sugarcane leaves were collected at various time intervals. Samples of sugarcane juice were collected at harvest. Residues of fipronil and its metabolites were quantified by gas liquid chromatograph. The limit of quantification of fipronil and its metabolites was 0.01 mg/kg for sugarcane leaves and juice. Total residues of fipronil and its metabolites in sugarcane leaves after 7 days of its application at 75 and 300 g a.i./ha were 0.26 and 0.66 mg/kg, respectively. Residues could not be detected after 60 and 90 following fipronil application at either concentration. In sugarcane leaves, fipronil was found to be the main constituent, followed by its metabolites amide, desulfinyl, sulfone and sulfide. Samples of sugarcane juice did not reveal the presence of fipronil or its metabolites following its application at both the dosages at harvest.
PMID:24343262 Mandal K, Singh B; Bull Environ Contam Toxicol 92 (2): 220-4 (2014)
The enantioselective bioaccumulation and elimination of fipronil in Anodonta woodiana (A. woodiana) were studied and the main metabolites fipronil desulfinyl, fipronil sulfide and fipronil sulfone were determined. The acute toxicity of the enantiomers of fipronil and the three metabolites were also investigated. In the bioaccumulation process, fipronil in A. woodiana reached equilibrium after 11 days with BCF value of 0.2, and the enantiomeric fraction (EF) values showed that the bioaccumulation was enantioselective with enantioenrichment of S-fipronil. The degradation of fipronil in A. woodiana fitted first-order kinetics model with half-lives of the enantiomers were 5.8 d for R-fipronil and 7.6 d for S-fipronil, and the EF values decreasing from 0.5 gradually indicating the R-enantiomer was preferentially degraded. The degradation of single enantiomers was also performed and the results revealed a fast conversion of R-fipronil to S-fipronil by A. woodiana. The three metabolites were all detected in A. woodiana-water system, in which fipronil sulfone and fipronil sulfide had higher concentration levels. According to the 72-hr LC50 values, S-fipronil was much more toxic than the racemate and R-fipronil. Moreover, the metabolites were more toxic than the parent fipronil. The results suggested the individual enantiomers of chiral pollutants and the metabolites should be considered in the risk assessments.
PMID:27037470 Qu H et al; J Hazard Mater 312: 169-174 (2016)
Fipronil is a phenylpyrazole insecticide commonly used in residential and agricultural applications. To understand more about the potential risks for human exposure associated with fipronil, urine and serum from dosed Long Evans adult rats (5 and 10 mg/kg bw) were analyzed to identify metabolites as potential biomarkers for use in human biomonitoring studies. Urine from treated rats was found to contain seven unique metabolites, two of which had not been previously reported-M4 and M7 which were putatively identified as a nitroso compound and an imine, respectively. Fipronil sulfone was confirmed to be the primary metabolite in rat serum. The fipronil metabolites identified in the respective matrices were then evaluated in matched human urine (n=84) and serum (n=96) samples from volunteers with no known pesticide exposures. Although no fipronil or metabolites were detected in human urine, fipronilsulfone was present in the serum of approximately 25% of the individuals at concentrations ranging from 0.1 to 4 ng/mL. These results indicate that many fipronil metabolites are produced following exposures in rats and that fipronil sulfone is a useful biomarker in human serum. Furthermore, human exposure to fipronil may occur regularly and require more extensive characterization.
PMID:25687022 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5247556 McMahen RL et al; Environ Int 78: 16-23 (2015)
... In this study, the tissue distribution, the metabolic fate, and the elimination of fipronil was investigated in rats using radiolabeled fipronil. When a single oral dose of (14)C-fipronil (10 mg/kg b.w.) was given to rats, the proportion of dose eliminated in urine and feces 72 hr after dosing was ca 4% for each route. At the end of the experiment the highest levels of radioactivity were found in adipose tissue and adrenals. The main part of the radioactivity present in investigated tissues (adipose tissue, adrenals, liver, kidney, testes) was due to fipronil-sulfone. Five additional metabolites, isolated from urine were characterized by LC-MS/MS. Most of them are formed by the loss of the trifluoromethylsulphinyl group and subsequent hydroxylation and/or conjugation to glucuronic acid or sulfate. In conclusion, the retention of the metabolite fipronil sulfone in tissues following fipronil administration raises the question of the potential toxicity of this insecticide.
PMID:24016625 Cravedi JP et al; Chemosphere 93 (10): 2276-83 (2013)
For more Metabolism/Metabolites (Complete) data for Fipronil (15 total), please visit the HSDB record page.
Female rabbits, rats and mice were dosed orally by gavage with M&B 46,030 (fipronil technical) (purity: 95.4% (based upon information provided under record no. 261658)) for 14 days. Two groups of rabbits received 0.4 or 1.2 mg/kg/day and two groups each of rats and mice were dosed with 0.4 or 4.0 mg/kg/day of the test material (study data for the rabbits is presented in record no. 261658). ... The predominant metabolite which was recovered was M&B 46136. ... For the rabbit, the study authors estimated the elimination half-lives for M&B 46136 to be 11 and 10 days for blood and fat, respectively. For the rodents, the values were 5 and 6 to 7 days, respectively. The brain half-lives ranged from 4 (mouse) to 9 days (rat) for the 3 species. The liver half-life values for the metabolite ranged between 3 and 5 days. The half-life in the thyroid of the rodents was 5 days. For the rabbit, the concentrations of M&B 46030 in the thyroid were too variable to calculate an elimination half-life.
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, Fipronil, Chemical Code No. 3995, p.6 (November 22, 2016). Available from, as of June 19, 2018: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
Male and female CD rats were dosed orally by gavage with 4 or 40 mg/kg of Fipronil- (U-[(14)C] phenyl) (radiochemical purity > 99%). The specific activities of the dosing preparations were adjusted as required using unlabeled Fipronil (purity 99.4%). ... Elimination t1/2 estimates were 135 and 171 hours for 40 mg/kg males and females, compared to 183 and 245 hours for 4 mg/kg males and females.
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, Fipronil, Chemical Code No. 3995, p.6-7 (November 22, 2016). Available from, as of June 19, 2018: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
Two female New Zealand white rabbits, 5 female Sprague-Dawley rats and 10 female CD1 mice were dosed orally with 5 mg/kg of M&B 46030- [phenyl-U-(14)C] (radiochemical purity: 98.7%, specific activity: 45.1 uCi/mg). The specific activity of the dosing preparations was adjusted to approximately 5.8 to 5.9 uCi/mg, using unlabeled M&B 46030 (purity: 99.3%). Urine and feces were collected up to 168 hours post-dose. Blood was drawn from each animal at specified intervals up to 168 hours post-dose. ... For the pharmacokinetic parameters, the Cmax values in the blood were 0.31, 0.64 and 0.58 ug/g for the rabbit, rat, and mouse, respectively. The tmax times were 12, 9 and 4 hours post-dose for the rabbit, rat, and mouse, respectively. The reported t1/2 times were 14, 3, and 3 days for the rabbit, rat and mouse, respectively.
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, Fipronil, Chemical Code No. 3995, p.5 (November 22, 2016). Available from, as of June 19, 2018: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
Female rabbits, rats and mice were dosed orally by gavage with M&B 46,030 (fipronil technical) (purity: 95.4% (based upon information provided under record no. 261658)) for 14 days. Two groups of rabbits received 0.4 or 1.2 mg/kg/day and two groups each of rats and mice were dosed with 0.4 or 4.0 mg/kg/day of the test material (study data for the rabbits is presented in record no. 261658). ... For the rabbit, the study authors estimated the elimination half-lives for M&B 46136 to be 11 and 10 days for blood and fat, respectively. For the rodents, the values were 5 and 6 to 7 days, respectively. The brain half-lives ranged from 4 (mouse) to 9 days (rat) for the 3 species. The liver half-life values for the metabolite ranged between 3 and 5 days. The half-life in the thyroid of the rodents was 5 days. For the rabbit, the concentrations of M&B 46030 in the thyroid were too variable to calculate an elimination half-life.
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, Fipronil, Chemical Code No. 3995, p.6 (November 22, 2016). Available from, as of June 19, 2018: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
Pharmacokinetic investigations showed that the whole-blood half-life at the single low dose was 149-200 hr in male and female rats /administered (14)C-fipronil (labelled uniformly at the phenyl ring; radiochemical purity, >97%)/... .
FAO/WHO; Pesticide Residues in Food-Fipronil (1997). Available from, as of June 18, 2018: https://www.inchem.org/documents/jmpr/jmpmono/v097pr09.htm
Fipronil is a second-generation phenilpirazol insecticide that is used in agriculture and veterinary medicine for protection against fleas, ticks, ants, cockroaches and other pests. The insecticide blocks the chloride channels associated with the gamma-amino butyric acid (GABA) receptors in mammals and the chloride channels associated with the GABA and glutamate (Glu) receptors in insects.
PMID:26409903 Magalhaes JZ et al; Neurotoxicol Teratol 52 (Pt A): 11-6 (2015)
GABA-gated chloride channel antagonists.
Crop Protection Handbook Volume 100, Meister Media Worldwide, Willoughby, OH 2014, p. 304
MicroRNAs (miRNAs), which are a class of small noncoding RNAs, can modulate the expression of many protein-coding genes when an organism is exposed to an environmental chemical. We previously demonstrated that miR-155 was significantly downregulated in adult zebrafish (Danio rerio) in response to fipronil (5-amino-1-[2,6-dichloro-4-(trifluoromethyl) phenyl]-4-[(trifluoromethyl) sulphinyl]-1H-pyrazole-3-carbonitrile) exposure. However, the regulation of this miRNA's predicted target gene cyb561d2, which is a member of the cytochrome b561 (cyt b561) family involved in electron transfer, cell defence, and chemical stress, has not been experimentally validated to date. In this study, we evaluated the effects of fipronil on miR-155 and cyb561d2 in zebrafish. The expression of miR-155 was downregulated, whereas cyb561d2 was upregulated in both mRNA and protein level in a dose-dependent manner upon stimulation of fipronil. The dual luciferase report assay demonstrated that miR-155 interacted with cyb561d2 3'-untranslated regions (3'-UTR). The expression of cyb561d2 was reduced in both mRNA and protein levels when ZF4 cells were transfected with an miR-155 mimic, whereas its expression levels of both mRNA and protein were increased when endogenous miR-155 was inhibited by transfection with an miR-155 inhibitor. The results improved our understanding of molecular mechanism of toxicity upon fipronil exposure, and presents miR-155 as a potential novel toxicological biomarker for chemical exposure.
PMID:25532856 Huang H et al; Environ Toxicol 31 (7): 877-86 (2016)
Fipronil is the first phenylpyrazole insecticide widely used in controlling pests, including pyrethroid, organophosphate and carbamate insecticides. It is generally accepted that fipronil elicits neurotoxicity via interactions with GABA and glutamate receptors, although alternative mechanisms have recently been proposed. This study evaluates the genotoxicity of fipronil and its likely mode of action in Drosophila S2 cells, as an in vitro model. Fipronil administrated the concentration- and time-dependent S2 cell proliferation. Intracellular biochemical assays showed that fipronil-induced S2 cell apoptosis coincided with a decrease in the mitochondrial membrane potential and an increase reactive oxygen species generation, a significant decrease of Bcl-2 and DIAP1, and a marked augmentation of Cyt c and caspase-3. Because caspase-3 is the major executioner caspase downstream of caspase-9 in Drosophila, enzyme activity assays were used to determine the activities of caspase-3 and caspase-9. Our results indicated that fipronil effectively induced apoptosis in Drosophila S2 cells through caspase-dependent mitochondrial pathways.
PMID:25868821 Zhang B et al; Pestic Biochem Physiol 119: 81-9 (2015)
For more Mechanism of Action (Complete) data for Fipronil (7 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
60
PharmaCompass offers a list of Fipronil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fipronil manufacturer or Fipronil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fipronil manufacturer or Fipronil supplier.
PharmaCompass also assists you with knowing the Fipronil API Price utilized in the formulation of products. Fipronil API Price is not always fixed or binding as the Fipronil Price is obtained through a variety of data sources. The Fipronil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fipronil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fipronil, including repackagers and relabelers. The FDA regulates Fipronil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fipronil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fipronil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fipronil supplier is an individual or a company that provides Fipronil active pharmaceutical ingredient (API) or Fipronil finished formulations upon request. The Fipronil suppliers may include Fipronil API manufacturers, exporters, distributors and traders.
click here to find a list of Fipronil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fipronil CEP of the European Pharmacopoeia monograph is often referred to as a Fipronil Certificate of Suitability (COS). The purpose of a Fipronil CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Fipronil EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Fipronil to their clients by showing that a Fipronil CEP has been issued for it. The manufacturer submits a Fipronil CEP (COS) as part of the market authorization procedure, and it takes on the role of a Fipronil CEP holder for the record. Additionally, the data presented in the Fipronil CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Fipronil DMF.
A Fipronil CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Fipronil CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Fipronil suppliers with CEP (COS) on PharmaCompass.
Fipronil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fipronil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fipronil GMP manufacturer or Fipronil GMP API supplier for your needs.
A Fipronil CoA (Certificate of Analysis) is a formal document that attests to Fipronil's compliance with Fipronil specifications and serves as a tool for batch-level quality control.
Fipronil CoA mostly includes findings from lab analyses of a specific batch. For each Fipronil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fipronil may be tested according to a variety of international standards, such as European Pharmacopoeia (Fipronil EP), Fipronil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fipronil USP).
