
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
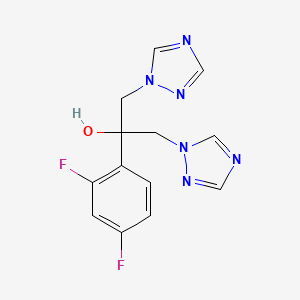

1. Apo Fluconazole
2. Apo-fluconazole
3. Bagyne
4. Diflucan
5. Fluc Hexal
6. Flucobeta
7. Flucolich
8. Fluconazol Abz
9. Fluconazol Al
10. Fluconazol Isis
11. Fluconazol Ratiopharm
12. Fluconazol Stada
13. Fluconazol Von Ct
14. Fluconazol-isis
15. Fluconazol-ratiopharm
16. Flunazul
17. Fungata
18. Lavisa
19. Loitin
20. Neofomiral
21. Oxifungol
22. Solacap
23. Triflucan
24. Uk 49858
25. Uk-49858
26. Uk49858
27. Zonal
1. 86386-73-4
2. Diflucan
3. Triflucan
4. Biozolene
5. Elazor
6. Biocanol
7. Fluconazol
8. Fungata
9. Zoltec
10. Fluconazolum
11. Flucostat
12. Difluconazole
13. 2-(2,4-difluorophenyl)-1,3-di(1h-1,2,4-triazol-1-yl)propan-2-ol
14. 2-(2,4-difluorophenyl)-1,3-bis(1h-1,2,4-triazol-1-yl)propan-2-ol
15. Uk 49858
16. Uk-49858
17. 2-(2,4-difluorophenyl)-1,3-bis(1,2,4-triazol-1-yl)propan-2-ol
18. Alkanazole
19. Flucazol
20. Fluconazole [usan]
21. 123631-92-5
22. Pritenzol
23. Flukezol
24. Flunizol
25. Zonal
26. Chembl106
27. Nsc-758661
28. 2-(2,4-difluorophenyl)-1,3-bis(1h-1,2,4-triazol-1-yl)-2-propanol
29. 8vzv102jfy
30. 2,4-difluoro-alpha,alpha-bis(1h-1,2,4-triazol-1-ylmethyl)benzyl Alcohol
31. 1h-1,2,4-triazole-1-ethanol, Alpha-(2,4-difluorophenyl)-alpha-(1h-1,2,4-triazol-1-ylmethyl)-
32. Uk-49,858
33. Alflucoz
34. Cryptal
35. Dimycon
36. Oxifugol
37. Canzol
38. Chebi:46081
39. Forcan
40. Syscan
41. Baten
42. Mutum
43. Zemyc
44. 2-(2,4-difluorophenyl)-1,3-di-1h-1,2,4-triazol-1-ylpropan-2-ol
45. Bayt006267
46. Bayt-006267
47. Alpha-(2,4-difluorophenyl)-alpha-(1h-1,2,4-triazol-1-ylmethyl)-1h-1,2,4-triazole-1-ethanol
48. Ncgc00095089-01
49. Flunazol
50. Fluconazol [spanish]
51. Fluconazolum [latin]
52. Loitin
53. Dsstox_cid_627
54. 2-(2,4-difluoro-phenyl)-1,3-bis-[1,2,4]triazol-1-yl-propan-2-ol
55. Dsstox_rid_75701
56. Dsstox_gsid_20627
57. Flc
58. Drg-0005
59. Diflazon
60. Fuconal
61. Triconal
62. Trican
63. 2-(2,4-difluorfenyl)-1,3-bis(1h-1,2,4-triazool-1-yl)propaan-2-ol
64. Diflucan (tn)
65. Smr000471882
66. Cas-86386-73-4
67. Flcz
68. Ccris 7211
69. Fluconazole & Hgcsf
70. Diflucan In Sodium Chloride 0.9%
71. Hsdb 7420
72. Sr-01000765440
73. Fluconazole In Sodium Chloride 0.9%
74. Unii-8vzv102jfy
75. Diflucan In Dextrose 5% In Plastic Container
76. Fluconazoli
77. Flucoral
78. Fluconazole & Mc-510,011
79. Fluconazole (f)
80. Fluconazole,(s)
81. 2,4-difluoro-,1-bis(1h-1,2,4-triazol-1-ylmethyl)benzyl Alcohol
82. Fluconazole In Dextrose 5% In Plastic Container
83. 2,4-difluoro-alpha,alpha-1-bis(1h-1,2,4-triazol-1-ylmethyl)benzyl Alcohol
84. Fluconazole- Bio-x
85. Fluzon [antifungal]
86. Mfcd00274549
87. Ks-1059
88. Fluconazole [usan:usp:inn:ban:jan]
89. Fluconazole In Sodium Chloride 0.9% In Plastic Container
90. Fluconazole In Combination With Mgcd290
91. Spectrum_001654
92. Diflucan In Sodium Chloride 0.9% In Plastic Container
93. Fluconazole [mi]
94. Spectrum2_001607
95. Spectrum3_001912
96. Spectrum4_000090
97. Spectrum5_001277
98. Fluconazole [inn]
99. Fluconazole [jan]
100. F0677
101. Fluconazole [hsdb]
102. Mgcd290 And Fluconazole
103. Fluconazole [vandf]
104. Cid_3365
105. Mg-3290 And Fluconazole
106. Schembl3151
107. Fluconazole [mart.]
108. Bspbio_003504
109. Fluconazole [usp-rs]
110. Fluconazole [who-dd]
111. Fluconazole [who-ip]
112. Kbiogr_000360
113. Kbioss_002134
114. Mls001066394
115. Mls001165780
116. Mls001195645
117. Mls001304713
118. Mls001306492
119. Mls006011884
120. Bidd:gt0799
121. Divk1c_001030
122. Spectrum1503975
123. Spbio_001613
124. Zinc4009
125. Fluconazole (jp17/usp/inn)
126. Dtxsid3020627
127. Bdbm25817
128. Hms503m21
129. Kbio1_001030
130. Kbio2_002134
131. Kbio2_004702
132. Kbio2_007270
133. Kbio3_003009
134. Fluconazole [orange Book]
135. Ninds_001030
136. Fluconazole [ep Monograph]
137. Hms1922o10
138. Hms2090i20
139. Hms2093m21
140. Hms2230o22
141. Hms3259h13
142. Hms3373i19
143. Hms3654p15
144. Hms3715f21
145. Hms3748g19
146. Pharmakon1600-01503975
147. Fluconazole [usp Monograph]
148. Fluconazoli [who-ip Latin]
149. Amy23415
150. Bcp28522
151. Fluconazole 2.0 Mg/ml In Methanol
152. Hy-b0101
153. 1,2,4-triazol-1-yl)propan-2-ol
154. Tox21_111419
155. Tox21_202240
156. Tox21_300581
157. Ac-428
158. Bbl005614
159. Ccg-39065
160. Dl-407
161. Fluconazole & Human Recombinant Granulocyte Colony Stimulating Factor
162. Nsc754343
163. Nsc758661
164. S1331
165. Stk619301
166. Akos000280854
167. Tox21_111419_1
168. Cs-1835
169. Db00196
170. Fluconazole 100 Microg/ml In Methanol
171. Fluconazole, >=98% (hplc), Powder
172. Nc00650
173. Nsc 758661
174. Nsc-754343
175. Idi1_001030
176. Ncgc00095089-02
177. Ncgc00095089-04
178. Ncgc00095089-05
179. Ncgc00095089-06
180. Ncgc00095089-07
181. Ncgc00095089-08
182. Ncgc00095089-09
183. Ncgc00095089-10
184. Ncgc00095089-11
185. Ncgc00254412-01
186. Ncgc00259789-01
187. 2-(2,4-difluorophenyl)-1,3-di(1h-
188. Bf164466
189. Fluconazole 100 Microg/ml In Acetonitrile
190. Sbi-0051880.p002
191. Uk-049858
192. Ft-0626437
193. Sw199616-2
194. En300-53634
195. C07002
196. D00322
197. Ab00052399-07
198. Ab00052399-08
199. Ab00052399_09
200. Ab00052399_10
201. 386f734
202. A841625
203. Q411478
204. Q-201120
205. Sr-01000765440-2
206. Sr-01000765440-4
207. Sr-01000765440-8
208. Uk-49858;uk 49858;uk49858
209. Brd-k05977355-001-02-6
210. Brd-k05977355-001-09-1
211. F2173-0496
212. Z235354561
213. Fluconazole, European Pharmacopoeia (ep) Reference Standard
214. 2-(2,4-difluorophenyl)-1,3-bis(1,2,4-triazol-1-yl)-2-propanol
215. Fluconazole, United States Pharmacopeia (usp) Reference Standard
216. 2-[2,4-bis(fluoranyl)phenyl]-1,3-bis(1,2,4-triazol-1-yl)propan-2-ol
217. Fluconazole, Pharmaceutical Secondary Standard; Certified Reference Material
218. 2,4-difluoro-1',1'-bis(1h-1,2,4-triazol-1-ylmethyl)benzyl Alcohol
219. A-(2,4-difluorophenyl)-a-(1h-1,2,4-triazol-1- Ylmethyl)-1h-1,2,4-triazole-1-ethanol
220. Fluconazole For Peak Identification, European Pharmacopoeia (ep) Reference Standard
221. Fluconazole Solution, 2.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
222. .alpha.-(2,4-difluorophenyl)-.alpha.-(1h-1,2,4-triazol-1-ylmethyl)-1h-1,2,4-triazole-1-ethanol
223. 1h-1,2,4-triazole-1-ethanol, .alpha.-(2,4-difluorophenyl)-.alpha.-(1h-1,2,4-triazol-1-ylmethyl)-
224. 1h-1,2,4-triazole-1-ethanol, 1-(2,4-difluorophenyl)-1-(1h-1,2,4-triazol-1-ylmethyl)-
| Molecular Weight | 306.27 g/mol |
|---|---|
| Molecular Formula | C13H12F2N6O |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 306.10406535 g/mol |
| Monoisotopic Mass | 306.10406535 g/mol |
| Topological Polar Surface Area | 81.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 358 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Diflucan |
| PubMed Health | Fluconazole |
| Drug Classes | Antifungal |
| Drug Label | DIFLUCAN (fluconazole), the first of a new subclass of synthetic triazole antifungal agents, is available as tablets for oral administration, as a powder for oral suspension, and as a sterile solution for intravenous use in glass and in Viaflex P... |
| Active Ingredient | Fluconazole |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 200mg/5ml; 200mg; 100mg; 50mg; 150mg; 50mg/5ml |
| Market Status | Prescription |
| Company | Pfizer |
| 2 of 4 | |
|---|---|
| Drug Name | Fluconazole |
| PubMed Health | Fluconazole |
| Drug Classes | Antifungal |
| Drug Label | Fluconazole USP, the first of a new subclass of synthetic triazole antifungal agents, is available as tablets for oral administration. Fluconazole USP is designated chemically as 2,4-difluoro-,1-bis(1H-1,2,4-triazol-1-ylmethyl) benzyl alcohol wit... |
| Active Ingredient | Fluconazole |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 200mg/5ml; 200mg; 150mg; 50mg/5ml; 100mg; 50mg |
| Market Status | Prescription |
| Company | Ranbaxy; Teva; Apotex; Aurobindo Pharma; Taro; Unique Pharm Labs; Roxane; Glenmark Generics; Ivax Sub Teva Pharms; Aurobindo Pharm; Dr Reddys Labs; Mylan |
| 3 of 4 | |
|---|---|
| Drug Name | Diflucan |
| PubMed Health | Fluconazole |
| Drug Classes | Antifungal |
| Drug Label | DIFLUCAN (fluconazole), the first of a new subclass of synthetic triazole antifungal agents, is available as tablets for oral administration, as a powder for oral suspension, and as a sterile solution for intravenous use in glass and in Viaflex P... |
| Active Ingredient | Fluconazole |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 200mg/5ml; 200mg; 100mg; 50mg; 150mg; 50mg/5ml |
| Market Status | Prescription |
| Company | Pfizer |
| 4 of 4 | |
|---|---|
| Drug Name | Fluconazole |
| PubMed Health | Fluconazole |
| Drug Classes | Antifungal |
| Drug Label | Fluconazole USP, the first of a new subclass of synthetic triazole antifungal agents, is available as tablets for oral administration. Fluconazole USP is designated chemically as 2,4-difluoro-,1-bis(1H-1,2,4-triazol-1-ylmethyl) benzyl alcohol wit... |
| Active Ingredient | Fluconazole |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 200mg/5ml; 200mg; 150mg; 50mg/5ml; 100mg; 50mg |
| Market Status | Prescription |
| Company | Ranbaxy; Teva; Apotex; Aurobindo Pharma; Taro; Unique Pharm Labs; Roxane; Glenmark Generics; Ivax Sub Teva Pharms; Aurobindo Pharm; Dr Reddys Labs; Mylan |
Mesh Heading: Antifungal agents
National Library of Medicine, SIS; ChemIDplus Record for Fluconazole (86386-73-4). Available from, as of April 17, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION: Antifungal; Orally active bistriazole antifungal agent
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 728-9
MEDICATION (VET): Used to treat systemic mycoses, particularly CNS-related conditions in dogs.
Milne, G.W.A. Veterinary Drugs: Synonyms and Properties. Ashgate Publishing Limited, Aldershot, Hampshire, England 2002., p. 80
Fluconazole ... /is/ indicated for the prophylaxis of febrile neutropenia in patients with hematologic malignancies. /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 335
For more Therapeutic Uses (Complete) data for FLUCONAZOLE (16 total), please visit the HSDB record page.
Although serious adverse hepatic effects have been reported only rarely with fluconazole, the possibility that these effects may occur during fluconazole therapy should be considered. Fluconazole therapy should be discontinued if signs and symptoms consistent with liver disease develop. If abnormal liver function test results occur during fluconazole therapy, the patient should be monitored for the development of more severe hepatic injury.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 507
Serious hepatic reactions (eg, necrosis, clinical hepatitis, cholestasis, fulminant hepatic failure) have been reported rarely in patients receiving fluconazole therapy. The manufacturer states that a clear relationship between these hepatic effects and daily dosage, duration of therapy, gender, or age has not been demonstrated. While hepatotoxicity usually has been reversible, fatalities have been reported. Fatalities principally have occurred in patients with serious underlying disease (eg, AIDS, malignancy) who were receiving fluconazole concomitantly with other drugs; however, at least one fatality involved an immunocompetent geriatric individual with renal impairment who developed fulminant hepatic necrosis within 10 days after fluconazole therapy was initiated.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 507
Mild, transient increases (1.5-3 times the upper limit of normal) in serum concentrations of AST (SGOT), ALT (SGPT), alkaline phosphatase, gamma-glutamyltransferase (GGT, gamma-glutamyl transpeptidase, GGTP), and bilirubin have been reported in about 5-7% of patients receiving fluconazole. In most reported cases, concentrations returned to pretreatment levels either during or after fluconazole therapy and were not associated with hepatotoxicity. However, higher increases in serum transaminase concentrations (8 or more times the upper limit of normal), which required discontinuance of the drug, have been reported in about 1% of patients receiving fluconazole. Any patient who develops abnormal liver function test results while receiving fluconazole should be closely monitored for the development of more severe hepatic injury.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 507
Because potentially fatal exfoliative skin disorders have been reported rarely in patients with a serious underlying disease receiving fluconazole, the possibility that these effects can occur should be considered. Immunocompromised patients (e.g., patients with HIV infections) who develop rash during fluconazole therapy should be monitored closely and the drug discontinued if the lesions progress.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 507
For more Drug Warnings (Complete) data for FLUCONAZOLE (17 total), please visit the HSDB record page.
Fluconazole can be administered in the treatment of the following fungal infections: 1) Vaginal yeast infections caused by Candida 2) Systemic Candida infections 3) Both esophageal and oropharyngeal candidiasis 4) Cryptococcal meningitis 5) UTI (urinary tract infection) by Candida 6) Peritonitis (inflammation of the peritoneum) caused by Candida **A note on fungal infection prophylaxis** Patients receiving bone marrow transplantation who are treated with cytotoxic chemotherapy and/or radiation therapy may be predisposed to candida infections, and may receive fluconazole as prophylactic therapy. **A note on laboratory testing** Obtaining specimens for fungal culture and other important laboratory studies such as serology or pathology is advised before starting fluconazole therapy in order to isolate the organisms to be eliminated through treatment. It is permissible to start therapy before the results are available, however, adjusting the therapy once laboratory results confirm the causative organism may be necessary.
FDA Label
Fluconazole has been demonstrated to show fungistatic activity against the majority of strains of the following microorganisms, curing fungal infections: _Candida albicans, Candida glabrata (Many strains are intermediately susceptible), Candida parapsilosis, Candida tropicalis, Cryptococcus neoformans_ This is achieved through steroidal inhibition in fungal cells, interfering with cell wall synthesis and growth as well as cell adhesion, thereby treating fungal infections and their symptoms. The fungistatic activity of fluconazole has also been shown in normal and immunocompromised animal models with both systemic and intracranial fungal infections caused by _Cryptococcus neoformans_ and for systemic infections caused by Candida albicans. It is important to note that resistant organisms have been found against various strains of organisms treated with fluconazole. This further substantiates the need to perform susceptibility testing when fluconazole is considered as an antifungal therapy. **A note on steroidal effects of fluconazole** There has been some concern that fluconazole may interfere with and inactivate human steroids/hormones due to the inhibition of hepatic cytochrome enzymes. Fluconazole has demonstrated to be more selective for _fungal_ cytochrome P-450 enzymes than for a variety of mammalian cytochrome P-450 enzymes. Fluconazole 50 mg administered daily for up to 28 days in individuals of reproductive age has been show to have no effect on testosterone plasma concentrations of males and plasma concentrations of steroids in females. A 200-400 mg dose of fluconazole showed no clinically relevant effect on steroid levels or on ACTH-stimulated steroid response in healthy males, in one clinical study mentioned on the European Medicines Agency label. Other studies have shown no significant effects of fluconazole on steroid levels, further confirming these data.
Cytochrome P-450 CYP2C19 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2C19. (See all compounds classified as Cytochrome P-450 CYP2C19 Inhibitors.)
14-alpha Demethylase Inhibitors
Compounds that specifically inhibit STEROL 14-DEMETHYLASE. A variety of azole-derived ANTIFUNGAL AGENTS act through this mechanism. (See all compounds classified as 14-alpha Demethylase Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Cytochrome P-450 CYP2C9 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2C9. (See all compounds classified as Cytochrome P-450 CYP2C9 Inhibitors.)
J02AC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AC - Imidazole and triazole derivatives
D01AC15 - Fluconazole
J - Antiinfectives for systemic use
J02 - Antimycotics for systemic use
J02A - Antimycotics for systemic use
J02AC - Triazole and tetrazole derivatives
J02AC01 - Fluconazole
Absorption
The pharmacokinetic properties of fluconazole are comparable after administration by the intravenous (IV) and oral (PO) routes. In healthy volunteers, the bioavailability of orally administered fluconazole is measured to be above 90%. It is extensively absorbed in the gastrointestinal tract when an oral dose is taken. Oral absorption is not affected by food intake with fluconazole but may increase the time until the maximum concentration is reached. Tmax (or the time taken to achieve the maximum concentration) in one clinical study of healthy patients receiving 50 mg/kg of fluconazole was 3 hours. Peak plasma concentrations (Cmax) in fasting and healthy volunteers occur between 1-2 hours post-dose. Steady-state concentrations are achieved within 5 to 10 days after oral doses of 50-400 mg administered once daily. Administration of a loading dose on the first day of fluconazole treatment, or twice the usual daily dose, leads to plasma concentrations close to steady-state by the second day. Mean AUC (area under the curve) was 20.3 in healthy volunteers receiving 25 mg of fluconazole. **A note on the capsule and powder form and malabsorption syndromes** The capsule forms of fluconazole often contain lactose and should not be administered with hereditary galactose intolerance, _Lapp lactase enzyme_ deficiency, or malabsorption of glucose/galactose. The powder form, used for the oral suspension, lists sucrose as an ingredient and should not be used in patients who have been diagnosed with fructose, glucose/galactose malabsorption, and _sucrase-isomaltase_ enzyme deficiency.
Route of Elimination
In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose measured in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.. A study of a 50mg radiolabeled dose of fluconazole revealed that 93.3% of the dose was found excreted in the urine. **A note on renal failure** The pharmacokinetics of fluconazole are significantly affected by renal dysfunction. The dose of fluconazole may need to be reduced in patients with decreased renal function. A 3-hour hemodialysis treatment lowers plasma fluconazole concentrations by about 50%.
Volume of Distribution
The apparent volume of distribution is said to be similar to the volume of distribution of total body water. One clinical study of healthy volunteers administered 50 mg/kg of fluconazole was 39L, based on a body weight of 60kg. Fluconazole shows substantial penetration in many body fluids, which is a property that renders it an ideal treatment for systemic fungal infections, especially when administered over a longer time. Fluconazole is found in high concentrations in the stratum corneum and dermis-epidermis of skin, in addition to eccrine sweat. Fluconazole is found to accumulate especially well in the stratum corneum, which is beneficial in superficial fungal infections. Saliva and sputum concentrations of fluconazole are found to be similar to the plasma concentrations. In patients diagnosed with fungal meningitis, fluconazole CSF (cerebrospinal fluid) levels are measured to be about 80% of the corresponding plasma levels. Therefore, fluconazole crosses the blood-brain barrier. The meninges are increasingly permeable to fluconazole in states of inflammation, facilitating treatment in meningitis.
Clearance
This drug is mainly eliminated by the kidneys and the mean body clearance in adults is reported to be 0.23 mL/min/kg. One clinical study of healthy subjects showed total clearance of 19.5 4.7 mL/min and renal clearance of 14.7 3.7 mL/min (1.17 0.28 and 0.88 0.22 L/h). Clearance in the pediatric population varies according to age, as does clearance in patients with renal failure.
The pharmacokinetics of fluconazole are similar following IV or oral administration. The drug is rapidly and almost completely absorbed from the GI tract, and there is no evidence of first-pass metabolism. Oral bioavailability of fluconazole exceeds 90% in healthy, fasting adults; peak plasma concentrations of the drug generally are attained within 1-2 hours after oral administration. ... The rate and extent of GI absorption of fluconazole are not affected by food. The manufacturer states that the commercially available fluconazole suspensions are bioequivalent to the 100-mg fluconazole tablets.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 511
Peak plasma fluconazole concentrations and AUCs increase in proportion to the dose over the oral dosage range of 50-400 mg. Steady-state plasma concentrations of fluconazole are attained within 5-10 days following oral doses of 50-400 mg given once daily. ... When fluconazole therapy is initiated with a single loading dose equal to twice the usual daily dosage and followed by the usual dosage given once daily thereafter, plasma concentrations of the drug reportedly approach steady state by the second day of therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 511
In healthy, fasting adults who received a single 1-mg/kg oral dose of fluconazole, peak plasma concentrations of the drug averaged 1.4 mcg/mL. Following oral administration of a single 400-mg dose of fluconazole in healthy, fasting adults, peak plasma concentrations average 6.72 mcg/mL (range: 4.12-8.1 mcg/mL).
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 511
In healthy adults receiving 50- or 100-mg doses of fluconazole given once daily by IV infusion over 30 minutes, serum concentrations of the drug 1 hour after dosing on the sixth or seventh day of therapy ranged from 2.14-2.81 or 3.86-4.96 mcg/mL, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 511
For more Absorption, Distribution and Excretion (Complete) data for FLUCONAZOLE (14 total), please visit the HSDB record page.
Fluconazole is metabolized minimally in the liver. Fluconazole is an inhibitor of CYP2C9, CYP3A4 and CYP2C19. Two metabolites were detected in the urine of healthy volunteers taking a 50 mg radiolabeled dose of fluconazole; a glucuronidated metabolite on the hydroxyl moiety (6.5%) and a fluconazole N-oxide metabolite (2%). The same study indicated that no signs of metabolic cleavage of fluconazole were observed, suggesting a difference in metabolism when compared to other agents in the same drug class, which are heavily metabolized in the liver.
Hepatic accounts for <10% of elimination
Lelkin, J.B., Paloucek, F.P., Poisoning & Toxicology Compendium. LEXI-COMP Inc. & American Pharmaceutical Association, Hudson, OH 1998., p. 280
The terminal elimination half-life in the plasma is approximately 30 hours (range: 20-50 hours) after oral administration. The long plasma elimination half-life supports a single dose therapy for vaginal candidiasis, once daily and once weekly dosing for other indications.. Patients with renal failure may require dosage adjustment, and half-life can be significantly increased in these patients.
The plasma elimination half-life of fluconazole in adults with normal renal function is approximately 30 hours (range: 20-50 hours). In one study, plasma elimination half-life of the drug was 22 hours after the first day of therapy and 23.8 and 28.6 hours after 7 and 26 days of therapy, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 511
In a limited, single-dose study in HIV-infected adults, the plasma elimination half-life of fluconazole averaged 32 hours (range: 25-42 hours) in those with absolute helper/inducer (CD4+, T4+) T-cell counts greater than 200 cu m and 50 hours (range: 32-69 hours) in those with CD4+ T-cell counts less than 200 cu m. In other single-dose studies in a limited number of HIV-infected adults with CD4+ T-cell counts less than 200 cu m, the plasma elimination half-life of the drug averaged 35-40 hours (range 22-75 hours).
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 511
The mean plasma half-life of fluconazole in children 9 months to 15 years of age has ranged from about 15-25 hours. In a limited study in premature neonates who received IV fluconazole once every 72 hours, the plasma half-life decreased over time, averaging 88 hours after the first dose and 55 hours after the fifth dose (day 13).
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 511
Fluconazole is a very selective inhibitor of fungal cytochrome P450 dependent enzyme _lanosterol 14--demethylase_. This enzyme normally works to convert _lanosterol_ to _ergosterol_, which is necessary for fungal cell wall synthesis. The free nitrogen atom located on the azole ring of fluconazole binds with a single iron atom located in the heme group of lanosterol 14--demethylase. This prevents oxygen activation and, as a result, inhibits the demethylation of lanosterol, halting the process of ergosterol biosynthesis. Methylated sterols are then found to accumulate in the fungal cellular membrane, leading to an arrest of fungal growth. These accumulated sterols negatively affect the structure and function of the fungal cell plasma membrane. Fluconazole resistance may arise from an alteration in the amount or function of the target enzyme (lanosterol 14--demethylase), altered access to this enzyme, or a combination of the above. Other mechanisms may also be implicated, and studies are ongoing.
Fluconazole usually is fungistatic in action. Fluconazole and other triazole-derivative antifungal agents (e.g., itraconazole, terconazole) appear to have a mechanism of action similar to that of the imidazole-derivative antifungal agents (e.g., butoconazole, clotrimazole, econazole, ketoconazole, miconazole, oxiconazole). Like imidazoles, fluconazole presumably exerts its antifungal activity by altering cellular membranes resulting in increased membrane permeability, leakage of essential elements (eg, amino acids, potassium), and impaired uptake of precursor molecules (eg, purine and pyrimidine precursors to DNA). Although the exact mechanism of action of fluconazole and other triazoles has not been fully determined, the drugs inhibit cytochrome P-450 14-a-desmethylase in susceptible fungi, which leads to accumulation of C-14 methylated sterols (e.g., lanosterol) and decreased concentrations of ergosterol. It appears that this may occur because a nitrogen atom (N-4) in the triazole molecule binds to the heme iron of cytochrome P-450 14-a-desmethylase in susceptible fungi. Unlike some imidazoles (eg, clotrimazole, econazole, miconazole, oxiconazole) that suppress ATP concentrations in intact cells and spheroplasts of C. albicans, fluconazole does not appear to have an appreciable effect on ATP concentrations in the organism. It is unclear whether this effect is related to the in vivo antifungal effects of the drugs. Fluconazole generally is fungistatic against Candida albicans when the organism is in either the stationary or early logarithmic phase of growth.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 509
Fungistatic; may be fungicidal, depending on the concentration; azole antifungals interfere with cytochrome P450 enzyme activity, which is necessary for the demethylation of 14-alpha-methylsterols to ergosterol. Ergosterol, the principal sterol in the fungal cell membrane, becomes depleted. This damages the cell membrane, producing alterations in membrane functions and permeability. In Candida albicans, azole antifungals inhibit transformation of blastospores into invasive mycelial form.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 336
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2015-09-04
Pay. Date : 2015-04-30
DMF Number : 24437
Submission : 2010-12-10
Status : Active
Type : II

Certificate Number : CEP 2009-364 - Rev 01
Issue Date : 2023-09-18
Type : Chemical
Substance Number : 2287
Status : Valid

Registration Number : 222MF10167
Registrant's Address : B-4, IDA, Gandhinagar, Hyderabad-500037, Telangana, INDIA
Initial Date of Registration : 2010-06-08
Latest Date of Registration : --

Date of Issue : 2022-09-16
Valid Till : 2025-07-02
Written Confirmation Number : WC-0191
Address of the Firm :

NDC Package Code : 51686-0006
Start Marketing Date : 2007-10-16
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Hanyoung Farm Co., Ltd.
Registration Date : 2024-02-16
Registration Number : 20140108-74-A-296-21(7)
Manufacturer Name : Virupaksha Organics Limited
Manufacturer Address : Sy.No.10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District-502 319, Telangana State, India

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14714
Submission : 1999-12-15
Status : Active
Type : II

Certificate Number : R1-CEP 2007-219 - Rev 02
Issue Date : 2019-06-14
Type : Chemical
Substance Number : 2287
Status : Valid

NDC Package Code : 64567-0002
Start Marketing Date : 2011-06-07
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2005-08-31
Registration Number : 20050831-74-A-158-11
Manufacturer Name : INKE SA
Manufacturer Address : Area Industrial del Llobregat, C/Argent 1, 08755 Castellbisbal Barcelona
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.

Date of Issue : 2022-06-17
Valid Till : 2025-07-07
Written Confirmation Number : WC-0067
Address of the Firm :

NDC Package Code : 55111-039
Start Marketing Date : 2003-01-27
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38391
Submission : 2023-06-16
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2015-09-04
Pay. Date : 2015-04-30
DMF Number : 24437
Submission : 2010-12-10
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14714
Submission : 1999-12-15
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15822
Submission : 2002-01-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11524
Submission : 1995-05-24
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-10-07
Pay. Date : 2015-09-29
DMF Number : 15161
Submission : 2000-11-27
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15883
Submission : 2002-03-04
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2021-03-29
Pay. Date : 2021-03-24
DMF Number : 8975
Submission : 1991-02-01
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-10-04
Pay. Date : 2013-09-25
DMF Number : 15045
Submission : 2000-08-11
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14863
Submission : 2000-05-02
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Certificate Number : CEP 2009-364 - Rev 01
Status : Valid
Issue Date : 2023-09-18
Type : Chemical
Substance Number : 2287
Certificate Number : R1-CEP 2007-219 - Rev 02
Status : Valid
Issue Date : 2019-06-14
Type : Chemical
Substance Number : 2287
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2015-233 - Rev 00
Status : Valid
Issue Date : 2022-03-16
Type : Chemical
Substance Number : 2287

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2007-141 - Rev 01
Status : Valid
Issue Date : 2018-01-04
Type : Chemical
Substance Number : 2287

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2010-265 - Rev 00
Status : Valid
Issue Date : 2017-01-11
Type : Chemical
Substance Number : 2287

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2007-100 - Rev 02
Status : Valid
Issue Date : 2022-12-05
Type : Chemical
Substance Number : 2287

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2007-308 - Rev 00
Status : Valid
Issue Date : 2014-12-02
Type : Chemical
Substance Number : 2287

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2010-247 - Rev 00
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2014-01-21
Type : Chemical
Substance Number : 2287

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2021-366 - Rev 00
Status : Valid
Issue Date : 2022-02-28
Type : Chemical
Substance Number : 2287

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2011-338 - Rev 00
Status : Withdrawn by Holder
Issue Date : 2020-07-31
Type : Chemical
Substance Number : 2287

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Registrant Name : Sanil Pharma Co., Ltd.
Registration Date : 2021-06-11
Registration Number : 20140108-74-A-296-21(1)
Manufacturer Name : Virupaksha Organics Limited
Manufacturer Address : Sy.No.10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District-502 319, Telanga...
Registrant Name : Hanyoung Farm Co., Ltd.
Registration Date : 2024-02-16
Registration Number : 20140108-74-A-296-21(7)
Manufacturer Name : Virupaksha Organics Limited
Manufacturer Address : Sy.No.10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District-502 319, Telanga...
Registrant Name : Sungjin Exim Co., Ltd.
Registration Date : 2021-12-06
Registration Number : 20140108-74-A-296-21(4)
Manufacturer Name : Virupaksha Organics Limited
Manufacturer Address : Sy.No.10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District-502 319, Telanga...
Registrant Name : Sanil Pharmaceutical Co., Ltd.
Registration Date : 2021-12-03
Registration Number : 20140108-74-A-296-21(3)
Manufacturer Name : Virupaksha Organics Limited
Manufacturer Address : Sy.No.10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District-502 319, Telanga...
Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2014-01-08
Registration Number : 20140108-74-A-296-21
Manufacturer Name : Virupaksha Organics Limited
Manufacturer Address : Sy.No.10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District-502 319, Telanga...
Registrant Name : Toru Corporation
Registration Date : 2023-06-19
Registration Number : 20140108-74-A-296-21(6)
Manufacturer Name : VIRUPAKSHA ORGANICS LTD
Manufacturer Address : Sy.No. 10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District - 502 319, Tela...
Registrant Name : Hwail Pharmaceutical Co., Ltd.
Registration Date : 2022-03-02
Registration Number : 20140108-74-A-296-21(5)
Manufacturer Name : Virupaksha Organics Limited
Manufacturer Address : Sy.No.10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District-502 319, Telanga...
Registrant Name : Ace Biopharm Co., Ltd.
Registration Date : 2021-07-07
Registration Number : 20140108-74-A-296-21(2)
Manufacturer Name : Virupaksha Organics Limited
Manufacturer Address : Sy.No.10, Gaddapothram Village, Jinnaram Mandal, Sangareddy District-502 319, Telanga...
Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2005-08-31
Registration Number : 20050831-74-A-158-11
Manufacturer Name : INKE SA
Manufacturer Address : Area Industrial del Llobregat, C/Argent 1, 08755 Castellbisbal Barcelona
Registrant Name : Narsha Farm Co., Ltd.
Registration Date : 2021-12-06
Registration Number : 20110615-74-A-243-18(3)
Manufacturer Name : Atul Bioscience Ltd.
Manufacturer Address : Plot No. N-37, Additional MIDC, Anand Nagar, Ambernath(E), Maharashtra State, India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
CAS Number : 288-88-0
End Use API : Fluconazole
About The Company : Chynops Pharma, a company certified with ISO 9001:2015, is situated in Ahmedabad, India. We specialize in exporting, supplying, and trading a wide range of prod...
CAS Number : 372-18-9
End Use API : Fluconazole
About The Company : Anupam Rasayan India Limited was started in 1976 and we are 36 years old. ISO 9001:2008 and ISO 14001:2004 certified chemical company with sound technology, en...

2,4-difluoro-alpha-(1h-1,2,4-triazolyl)acetophenon...
CAS Number : 86404-63-9
End Use API : Fluconazole
About The Company : Badrivishal Chemicals & Pharmaceuticals is a quality conscious manufacturer and exporter of Active Pharmaceutical Ingredients and Speciality Chemicals. Our mott...

CAS Number : 584-13-4
End Use API : Fluconazole
About The Company : Riddhi Pharma was set up in 1997 as a Manufacturer of API’s Intermediates and Fine Chemicals, especially in the field of Friedel Crafts Reaction / Bromination...

1[-2-(2,4-DIFLUOROPHENYL)-2,3-EPOXY PROPYL]-1H-1,2...
CAS Number : 86386-77-8
End Use API : Fluconazole
About The Company : Riddhi Pharma was set up in 1997 as a Manufacturer of API’s Intermediates and Fine Chemicals, especially in the field of Friedel Crafts Reaction / Bromination...

2,4-DIFLUORO-2-(1H-1,2,4-TRIAZOLE-1YL) ACETOPHENON...
CAS Number : 86404-63-9
End Use API : Fluconazole
About The Company : Riddhi Pharma was set up in 1997 as a Manufacturer of API’s Intermediates and Fine Chemicals, especially in the field of Friedel Crafts Reaction / Bromination...

CAS Number : 288-88-0
End Use API : Fluconazole
About The Company : Riddhi Pharma was set up in 1997 as a Manufacturer of API’s Intermediates and Fine Chemicals, especially in the field of Friedel Crafts Reaction / Bromination...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 200MG/100ML (2MG/ML)
Approval Date : 2004-07-29
Application Number : 76653
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 400MG/200ML (2MG/ML)
Approval Date : 2004-07-29
Application Number : 76653
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AB
Brand Name : FLUCONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date : 2004-07-29
Application Number : 76658
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AB
Brand Name : FLUCONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG
Approval Date : 2004-07-29
Application Number : 76658
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AB
Brand Name : FLUCONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 150MG
Approval Date : 2004-07-29
Application Number : 76658
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AB
Brand Name : FLUCONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Approval Date : 2004-07-29
Application Number : 76658
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 200MG/100ML (2MG/ML)
Approval Date : 2005-01-13
Application Number : 76837
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 400MG/200ML (2MG/ML)
Approval Date : 2005-01-13
Application Number : 76837
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : FLUCONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date : 2004-07-29
Application Number : 76507
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : FLUCONAZOLE
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : 50MG/5ML
Approval Date : 2006-12-18
Application Number : 76918
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Not Available
Brand Name : Disintequik™ MCC 25
Application : Co-Processed Excipients
Excipient Details : Direct Tabletting Operations Where Fast Disintegration Is Required
Dosage Form : Tablet
Grade : Not Available
Brand Name : Foremost Fast Flo® 316
Application : Fillers, Diluents & Binders
Excipient Details : Foremost Fast Flo® 316 is a pharmaceutical excipient used in direct compression.
Pharmacopoeia Ref : USP/NF, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Not Available
Brand Name : Foremost™ Lactose 313
Application : Fillers, Diluents & Binders
Excipient Details : Wet Granulation & Capsule Filling
Pharmacopoeia Ref : USP/NF, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Not Available
Brand Name : Foremost™ Lactose 315
Application : Fillers, Diluents & Binders
Excipient Details : Foremost™ Lactose 315 is a pharmaceutical excipient used in direct compression.
Pharmacopoeia Ref : USP/NF, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Not Available
Brand Name : Foremost® Lactose 310
Application : Fillers, Diluents & Binders
Excipient Details : Wet Granulation & Capsule Filling
Pharmacopoeia Ref : USP/NF, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Not Available
Brand Name : Foremost® Lactose 312
Application : Fillers, Diluents & Binders
Excipient Details : Wet Granulation & Capsule Filling
Pharmacopoeia Ref : USP/NF, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Not Available
Application : Co-Processed Excipients
Excipient Details : Direct Compression
Dosage Form : Tablet
Grade : Not Available
Application : Co-Processed Excipients
Excipient Details : Direct Compression
Pharmacopoeia Ref : NF/EP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Not Available
Application : Co-Processed Excipients
Excipient Details : Standard Direct Tabletting Or Roller Compaction
Dosage Form : Tablet
Grade : Not Available
Application : Co-Processed Excipients
Excipient Details : Direct Compression
Pharmacopoeia Ref : NF/EP/JP
Technical Specs : Not Available
Ingredient(s) : Spray Dried Monohydrate Lactose
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?