
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
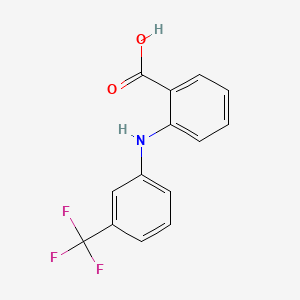

1. Acid, Flufenamic
2. Dignodolin
1. 530-78-9
2. Arlef
3. Fluphenamic Acid
4. Nichisedan
5. Achless
6. 2-((3-(trifluoromethyl)phenyl)amino)benzoic Acid
7. Flufacid
8. Fullsafe
9. Lanceat
10. Paraflu
11. Plostene
12. Tecramine
13. Parlef
14. Parlif
15. Surika
16. Flufenaminsaeure
17. N-(3-trifluoromethylphenyl)anthranilic Acid
18. Reumajust A
19. Acido Flufenamico
20. Ristogen
21. Sastridex
22. Ansatin
23. Meralen
24. 2-{[3-(trifluoromethyl)phenyl]amino}benzoic Acid
25. Fluore-200
26. Ci 440
27. Nsc-82699
28. Ant-1
29. Benzoic Acid, 2-[[3-(trifluoromethyl)phenyl]amino]-
30. 3'-trifluoromethyldiphenylamine-2-carboxylic Acid
31. Acide Flufenamique
32. Cn-27,554
33. Acidum Flufenamicum
34. Tvx 916
35. Inf 1837
36. 2-[[3-(trifluoromethyl)phenyl]amino]benzoic Acid
37. 2-[3-(trifluoromethyl)anilino]benzoic Acid
38. C.i. 440
39. Inf-1837
40. Nsc 219007
41. Benzoic Acid, 2-((3-(trifluoromethyl)phenyl)amino)-
42. N-(alpha,alpha,alpha-trifluoro-m-tolyl)anthranilic Acid
43. 2-((3-trifluromethyl)phenyl)aminobenzoic Acid
44. 2-[[3-(trifluoromethyl)phenyl]amino] Benzoic Acid
45. N-(m-trifluoromethylphenyl)-2-aminobenzoic Acid
46. Nsc-219007
47. 60gcx7y6bh
48. Chembl23588
49. Mls000028563
50. Chebi:42638
51. N-[3-(trifluoromethyl)phenyl]anthranilic Acid
52. Saal-f
53. Ci-440
54. Ncgc00016490-06
55. Cas-530-78-9
56. Cn-27554
57. Smr000059027
58. N-(3-trifluoromethylphenyl)-anthranilic Acid
59. Dsstox_cid_3063
60. Wln: Qvr Bmr Cxfff
61. Dsstox_rid_76859
62. Dsstox_gsid_23063
63. Ffa
64. Flufenamicacid
65. N-(.alpha.,.alpha.,.alpha.-trifluoro-m-tolyl)anthranilic Acid
66. Flufenaminsaeure [german]
67. Acido Flufenamico [italian]
68. Flf
69. Ccris 5266
70. 2-[(3-trifluromethyl)phenyl]aminobenzoic Acid
71. N-[(3-trifluoromethyl)phenyl]anthranilic Acid
72. Acide Flufenamique [inn-french]
73. Acido Flufenamico [inn-spanish]
74. Acidum Flufenamicum [inn-latin]
75. N-((m-trifluoromethyl)phenyl)-2-aminobenzoic Acid
76. N-[(m-trifluoromethyl)phenyl]-2-aminobenzoic Acid
77. N-[m-(trifluoromethyl)phenyl]-2-aminobenzoic Acid
78. Sr-01000000241
79. Einecs 208-494-1
80. Anthranilic Acid,.alpha.,.alpha.-trifluoro-m-tolyl)-
81. N-(.alpha.,.alpha.-trifluoro-m-tolyl)anthranilic Acid
82. Unii-60gcx7y6bh
83. Flufenamic Acid [usan:inn:ban:jan]
84. Brn 1996069
85. Flufenamic-acid
86. Prestwick_220
87. Mfcd00002422
88. Arlef (tn)
89. Spectrum_001257
90. 1bm7
91. 2-(3-trifluoromethylanilino)benzoic Acid
92. Opera_id_578
93. Prestwick0_000203
94. Prestwick1_000203
95. Prestwick2_000203
96. Prestwick3_000203
97. Spectrum2_000789
98. Spectrum3_001273
99. Spectrum4_000102
100. Spectrum5_000686
101. Ec 208-494-1
102. Cbiol_001756
103. Schembl17497
104. Bspbio_000185
105. Bspbio_001319
106. Bspbio_002866
107. Cbdive_012649
108. Flufenamic Acid [mi]
109. Kbiogr_000039
110. Kbiogr_000424
111. Kbiogr_002267
112. Kbioss_000039
113. Kbioss_001737
114. Kbioss_002268
115. 3-14-00-00905 (beilstein Handbook Reference)
116. Mls001148610
117. Divk1c_000581
118. Flufenamic Acid [inn]
119. Flufenamic Acid [jan]
120. Spectrum1501015
121. Spbio_000898
122. Spbio_002106
123. Flufenamic Acid [usan]
124. Bpbio1_000205
125. Gtpl2447
126. Dtxsid7023063
127. Flufenamic Acid [mart.]
128. Bcbcmap01_000039
129. Bdbm17636
130. Hms501n03
131. Kbio1_000581
132. Kbio2_000039
133. Kbio2_001737
134. Kbio2_002267
135. Kbio2_002607
136. Kbio2_004305
137. Kbio2_004835
138. Kbio2_005175
139. Kbio2_006873
140. Kbio2_007403
141. Kbio3_000077
142. Kbio3_000078
143. Kbio3_002366
144. Kbio3_002747
145. Zinc86535
146. Anthranilic Acid, N-(alpha,alpha,alpha-trifluoro-m-tolyl)-
147. Flufenamic Acid [who-dd]
148. Cmap_000004
149. Ninds_000581
150. Bio1_000042
151. Bio1_000531
152. Bio1_001020
153. Bio2_000039
154. Bio2_000519
155. Flufenamic Acid (jan/usan/inn)
156. Hms1361b21
157. Hms1568j07
158. Hms1791b21
159. Hms1921b21
160. Hms1989b21
161. Hms2089e07
162. Hms2092b09
163. Hms2095j07
164. Hms2232g24
165. Hms3371f01
166. Hms3402b21
167. Hms3652f06
168. Hms3712j07
169. Hms3885p15
170. Pharmakon1600-01501015
171. Hy-b1221
172. Nsc82699
173. Tox21_110452
174. Tox21_302111
175. Ccg-40167
176. Nsc219007
177. Nsc757823
178. S4268
179. Stk985630
180. Akos000265536
181. Tox21_110452_1
182. Cs-4811
183. Db02266
184. Ks-1143
185. Nsc-757823
186. Idi1_000581
187. Idi1_033789
188. Ncgc00016490-01
189. Ncgc00016490-02
190. Ncgc00016490-03
191. Ncgc00016490-04
192. Ncgc00016490-05
193. Ncgc00016490-07
194. Ncgc00016490-08
195. Ncgc00016490-09
196. Ncgc00016490-10
197. Ncgc00016490-12
198. Ncgc00023200-03
199. Ncgc00023200-04
200. Ncgc00023200-05
201. Ncgc00023200-06
202. Ncgc00023200-07
203. Ncgc00255175-01
204. Cn-27544
205. Sbi-0051633.p002
206. Db-052254
207. 2-[3-(trifluoromethyl)anilino]-benzoic Acid
208. Ab00052198
209. Ft-0603454
210. N-(a,a,a-trifluoro-m-tolyl)anthranilic Acid
211. Sw196528-3
212. T2354
213. Unm000001246003
214. 2-[3-(trifluoromethyl)anilino]benzoic Acid #
215. 2-(3-(trifluoromethyl)phenylamino)benzoic Acid
216. 2-[(3-trifluoromethylphenyl)amino]benzoic Acid
217. A13333
218. D01581
219. D97458
220. Flufenamic Acid 100 Microg/ml In Acetonitrile
221. Ab00052198-14
222. Ab00052198_15
223. 530f789
224. Q416341
225. Sr-01000000241-2
226. Sr-01000000241-3
227. W-105772
228. Brd-k44067360-001-06-3
229. Brd-k44067360-001-16-2
230. F0909-0007
231. Flufenamic Acid, Analytical Standard, For Drug Analysis
232. Anthranilic Acid, N-(.alpha.,.alpha.,.alpha.-trifluoro-m-tolyl)-
| Molecular Weight | 281.23 g/mol |
|---|---|
| Molecular Formula | C14H10F3NO2 |
| XLogP3 | 5.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 281.06636305 g/mol |
| Monoisotopic Mass | 281.06636305 g/mol |
| Topological Polar Surface Area | 49.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 346 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AG - Fenamates
M01AG03 - Flufenamic acid

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
16
PharmaCompass offers a list of Flufenamic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Flufenamic Acid manufacturer or Flufenamic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Flufenamic Acid manufacturer or Flufenamic Acid supplier.
PharmaCompass also assists you with knowing the Flufenamic Acid API Price utilized in the formulation of products. Flufenamic Acid API Price is not always fixed or binding as the Flufenamic Acid Price is obtained through a variety of data sources. The Flufenamic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Flufenamic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Flufenamic Acid, including repackagers and relabelers. The FDA regulates Flufenamic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Flufenamic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Flufenamic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Flufenamic Acid supplier is an individual or a company that provides Flufenamic Acid active pharmaceutical ingredient (API) or Flufenamic Acid finished formulations upon request. The Flufenamic Acid suppliers may include Flufenamic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Flufenamic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Flufenamic Acid Drug Master File in Japan (Flufenamic Acid JDMF) empowers Flufenamic Acid API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Flufenamic Acid JDMF during the approval evaluation for pharmaceutical products. At the time of Flufenamic Acid JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Flufenamic Acid suppliers with JDMF on PharmaCompass.
A Flufenamic Acid written confirmation (Flufenamic Acid WC) is an official document issued by a regulatory agency to a Flufenamic Acid manufacturer, verifying that the manufacturing facility of a Flufenamic Acid active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Flufenamic Acid APIs or Flufenamic Acid finished pharmaceutical products to another nation, regulatory agencies frequently require a Flufenamic Acid WC (written confirmation) as part of the regulatory process.
click here to find a list of Flufenamic Acid suppliers with Written Confirmation (WC) on PharmaCompass.
Flufenamic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Flufenamic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Flufenamic Acid GMP manufacturer or Flufenamic Acid GMP API supplier for your needs.
A Flufenamic Acid CoA (Certificate of Analysis) is a formal document that attests to Flufenamic Acid's compliance with Flufenamic Acid specifications and serves as a tool for batch-level quality control.
Flufenamic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Flufenamic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Flufenamic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Flufenamic Acid EP), Flufenamic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Flufenamic Acid USP).

