
Synopsis
Synopsis
0
KDMF
0
VMF
0
Canada

0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
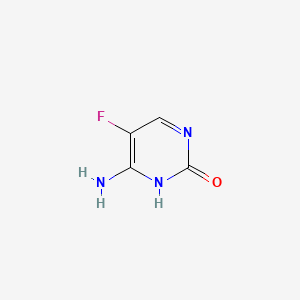

1. 5-fluorocytosine
2. Alcobon
3. Ancobon
4. Ancotil
1. 5-fluorocytosine
2. 2022-85-7
3. Ancobon
4. Ancotil
5. Fluorocytosine
6. 5-fluorocystosine
7. 5-fc
8. Flucytosin
9. Fluocytosine
10. 5-fluorocytosin
11. Fluorcytosine
12. Alcobon
13. 4-amino-5-fluoropyrimidin-2(1h)-one
14. Ro 2-9915
15. Flucytosinum
16. Cytosine, 5-fluoro-
17. 5-fluoro Cytosine
18. 4-amino-5-fluoro-2(1h)-pyrimidinone
19. 4-amino-5-fluoropyrimidin-2-ol
20. 2(1h)-pyrimidinone, 4-amino-5-fluoro-
21. 117969-88-7
22. 5-flurocytosine
23. 5-flucytosine
24. 2-hydroxy-4-amino-5-fluoropyrimidine
25. Ancobon (tn)
26. Ro 29915 E/265601
27. Nsc 103805
28. 6-amino-5-fluoro-1h-pyrimidin-2-one
29. Flucytosine (ancobon)
30. 4-amino-5-fluoro-2-hydroxypyrimidine
31. 130256-61-0
32. 2-pyrimidinol, 4-amino-5-fluoro- (9ci)
33. Ro-2-9915
34. Chebi:5100
35. Mfcd00006035
36. Ro-29915
37. Nsc-103805
38. Mls000069463
39. Flucitosina
40. 4-amino-2-hydroxy-5-fluoropyrimidine
41. 5-fluoro-4-imino-1,2,3,4-tetrahydropyrimidin-2-one
42. Nsc103805
43. Flucytosine [usan)
44. D83282dt06
45. 4-amino-5-fluoro-1h-pyrimidin-2-one
46. Ncgc00016599-01
47. Flucitosina [dcit]
48. Smr000059047
49. Cas-2022-85-7
50. 4-amino-5-fluoro-2(1h)-pyrimidine
51. Dsstox_cid_3059
52. Flucytosine [usan]
53. Dsstox_rid_76856
54. Dsstox_gsid_23059
55. Flucytosinum [inn-latin]
56. Flucytosone
57. Ancotyl
58. Hsdb 3082
59. 6-amino-2-oxo-5-fluoropyrimidine
60. Einecs 217-968-7
61. Flucytosina
62. Flourocytosine
63. Ancoban
64. 2(1h)-pyrimidinone, 4-amino-5-fluoro-)
65. 5-fluorocytocine
66. Unii-d83282dt06
67. Flucytosine,(s)
68. 5-fluoro-cytosine
69. 5 Fluoro Cytosine
70. 4-amino-5-fluoro-2-hyroxypyrimidine
71. 6-amino-5-fluoropyrimidin-2(1h)-one
72. Flucytosine (5-fc)
73. Flucytosine [usan:usp:inn:ban:jan]
74. Cpd000059047
75. St028644
76. 6-amino-5-fluoro-3-hydropyrimidin-2-one
77. Opera_id_178
78. 5-fluorocytosine Form I
79. Flucytosine [mi]
80. Prestwick0_000934
81. Prestwick1_000934
82. Prestwick2_000934
83. Prestwick3_000934
84. Flucytosine [inn]
85. Flucytosine [jan]
86. 5-fluorocytosine Form Ii
87. F0321
88. Flucytosine [hsdb]
89. Ec 217-968-7
90. Wln: T6mvnj Dz Ef
91. Chembl1463
92. Flucytosine [mart.]
93. Schembl24063
94. Bspbio_000868
95. Flucytosine [usp-rs]
96. Flucytosine [who-dd]
97. Flucytosine [who-ip]
98. Mls000759519
99. Mls001076503
100. Mls001424013
101. Flucytosine, 5-fluorocytosine
102. Spbio_003037
103. Bpbio1_000956
104. Flucytosine (jp17/usp/inn)
105. Dtxsid3023059
106. Schembl14696800
107. 4-amino-5-fluoro-2-pyrimidinol
108. Ala-ala-phep-nitroanilide
109. Flucytosine [orange Book]
110. Flucytosine For System Suitability
111. 2-pyrimidinol,4-amino-5-fluoro-
112. 4-amino-5-fluoro-pyrimidin-2-ol
113. Flucytosine [ep Monograph]
114. Flucytosine [usp Impurity]
115. Hms1570l10
116. Hms2051m04
117. Hms2093j05
118. Hms2097l10
119. Hms2233i14
120. Hms3373l05
121. Hms3393m04
122. Hms3655g17
123. Hms3714l10
124. Pharmakon1600-01505429
125. Zinc896546
126. 5-fluorocytosine [who-ip]
127. Flucytosine [usp Monograph]
128. 5-fluorocytosine, Nucleoside Analog
129. Bcp02877
130. Flucytosine 2.0 Mg/ml In Methanol
131. Flucytosinum [who-ip Latin]
132. Hy-b0139
133. Tox21_110515
134. Bdbm50248003
135. Mfcd00179326
136. Mfcd03547958
137. Nsc759130
138. S1666
139. Stk292386
140. Akos004912683
141. Akos005063821
142. Akos015896898
143. Akos030241326
144. Tox21_110515_1
145. Bcp9000692
146. Ccg-100837
147. Ccg-213434
148. Cs-1935
149. Db01099
150. Gs-5578
151. Ks-1060
152. Nc00087
153. Nsc-759130
154. Sb57097
155. Cytosine, 5-fluoro- (6ci,7ci,8ci)
156. Ncgc00016599-02
157. Ncgc00016599-04
158. Ncgc00016599-05
159. Ac-11748
160. Bp-30254
161. Emtricitabine Impurity E [who-ip]
162. Nci60_000093
163. Sy004438
164. Bcp0726000281
165. Db-005380
166. Ab00513969
167. Am20090149
168. Ft-0601273
169. Ft-0695664
170. Sw197278-3
171. 6-amino-5-fluoro-1,2-dihydropyrimidin-2-one
172. 22p857
173. D00323
174. F-3010
175. H10295
176. Ab00444223-16
177. Ab00513969-02
178. Ab00513969_03
179. Ab00513969_04
180. Flucytosine 100 Microg/ml In Acetonitrile:water
181. 5-fluorocytosine, Vetec(tm) Reagent Grade, 99%
182. A814346
183. Q238490
184. Sr-01000721885
185. Sr-01000721885-5
186. Brd-k82143716-001-15-7
187. 4-amino-5-fluoropyrimidin-2(1h)-one [who-ip]
188. 5-fluorocytosine, Antibiotic For Culture Media Use Only
189. 6-amino-5-fluoro-2(1h)-pyrimidinone;5-fluorocytosine
190. Flucytosine, European Pharmacopoeia (ep) Reference Standard
191. Flucytosine, United States Pharmacopeia (usp) Reference Standard
192. Flucytosine, Pharmaceutical Secondary Standard; Certified Reference Material
193. Flucytosine For System Suitability, European Pharmacopoeia (ep) Reference Standard
194. 1ld
| Molecular Weight | 129.09 g/mol |
|---|---|
| Molecular Formula | C4H4FN3O |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 129.03383992 g/mol |
| Monoisotopic Mass | 129.03383992 g/mol |
| Topological Polar Surface Area | 67.5 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 208 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Ancobon |
| PubMed Health | Flucytosine (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | Ancobon (flucytosine), an antifungal agent, is available as 250 mg and 500 mg capsules for oral administration. Each capsule also contains corn starch, lactose and talc. Gelatin capsule shells contain parabens (butyl, methyl, propyl) and sodium propi... |
| Active Ingredient | Flucytosine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Valeant |
| 2 of 4 | |
|---|---|
| Drug Name | Flucytosine |
| PubMed Health | Flucytosine (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | Flucytosine Capsules, USP are an antifungal agent available as 250 mg and 500 mg capsules for oral administration. Each capsule also contains corn starch, lactose monohydrate and talc. The 250 mg capsule shell contains FD and C Yellow No.6, FD and C... |
| Active Ingredient | Flucytosine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Sigmapharm Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Ancobon |
| PubMed Health | Flucytosine (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | Ancobon (flucytosine), an antifungal agent, is available as 250 mg and 500 mg capsules for oral administration. Each capsule also contains corn starch, lactose and talc. Gelatin capsule shells contain parabens (butyl, methyl, propyl) and sodium propi... |
| Active Ingredient | Flucytosine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Valeant |
| 4 of 4 | |
|---|---|
| Drug Name | Flucytosine |
| PubMed Health | Flucytosine (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | Flucytosine Capsules, USP are an antifungal agent available as 250 mg and 500 mg capsules for oral administration. Each capsule also contains corn starch, lactose monohydrate and talc. The 250 mg capsule shell contains FD and C Yellow No.6, FD and C... |
| Active Ingredient | Flucytosine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Sigmapharm Labs |
Antifungal Agents; Antimetabolites
National Library of Medicine's Medical Subject Headings. Citalopram. Online file (MeSH, 2018). Available from, as of February 2, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Flucytosine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 2, 2018: https://clinicaltrials.gov/
Flucytosine Capsules are indicated only in the treatment of serious infections caused by susceptible strains of Candida and/or Cryptococcus. Candida: Septicemia, endocarditis and urinary system infections have been effectively treated with flucytosine. Limited trials in pulmonary infections justify the use of flucytosine. Cryptococcus: Meningitis and pulmonary infections have been treated effectively. Studies in septicemias and urinary tract infections are limited, but good responses have been reported. /Included in US product label/
NIH; DailyMed. Current Medication Information for Flucytosine Capsule (Updated: January 8, 2018). Available from, as of February 8, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58189ec7-118b-458a-8e4f-7867a97cfcbd
Flucytosine Capsules should be used in combination with amphotericin B for the treatment of systemic candidiasis and cryptococcosis because of the emergence of resistance to Flucytosine Capsules. /Included in US product label/
NIH; DailyMed. Current Medication Information for Flucytosine Capsule (Updated: January 8, 2018). Available from, as of February 8, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58189ec7-118b-458a-8e4f-7867a97cfcbd
For more Therapeutic Uses (Complete) data for Flucytosine (10 total), please visit the HSDB record page.
/BOXED WARNING/ Use with extreme caution in patients with impaired renal function. Close monitoring of hematologic, renal and hepatic status of all patients is essential. These instructions should be thoroughly reviewed before administration of Flucytosine Capsules, USP.
NIH; DailyMed. Current Medication Information for Flucytosine Capsule (Updated: January 8, 2018). Available from, as of February 8, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58189ec7-118b-458a-8e4f-7867a97cfcbd
Flucytosine capsules must be given with extreme caution to patients with bone marrow depression. Patients may be more prone to depression of bone marrow function if they: 1) have a hematologic disease, 2) are being treated with radiation or drugs which depress bone marrow, or 3) have a history of treatment with such drugs or radiation. Bone marrow toxicity can be irreversible and may lead to death in immunosuppressed patients. Frequent monitoring of hepatic function and of the hematopoietic system is indicated during therapy.
NIH; DailyMed. Current Medication Information for Flucytosine Capsule (Updated: January 8, 2018). Available from, as of February 8, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58189ec7-118b-458a-8e4f-7867a97cfcbd
In addition to antiproliferative effects on the GI lining, adverse GI effects reported with flucytosine, which are sometimes severe, include anorexia, abdominal bloating, abdominal pain, diarrhea, dry mouth, duodenal ulcer, GI hemorrhage, nausea, vomiting, and ulcerative colitis.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 558
There are no adequate or controlled studies to date using flucytosine in pregnant women, and the drug should be used during pregnancy only when the potential benefits justify the possible risks to the fetus.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 559
For more Drug Warnings (Complete) data for Flucytosine (17 total), please visit the HSDB record page.
For the treatment (in combination with amphotericin B) of serious infections caused by susceptible strains of Candida (septicemia, endocarditis and urinary system infections) and/or Cryptococcus (meningitis and pulmonary infections).
FDA Label
Flucytosine is an antimetabolite that acts as an antifungal agent with in vitro and in vivo activity against Candida and Cryptococcus. Flucytosine enters the fungal cell via cytosine permease; thus, flucytosine is metabolized to 5-fluorouracil within fungal organisms. The 5-fluorouracil is extensively incorporated into fungal RNA and inhibits synthesis of both DNA and RNA. The result is unbalanced growth and death of the fungal organism. Antifungal synergism between Ancobon and polyene antibiotics, particularly amphotericin B, has been reported.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AE - Other antifungals for topical use
D01AE21 - Flucytosine
J - Antiinfectives for systemic use
J02 - Antimycotics for systemic use
J02A - Antimycotics for systemic use
J02AX - Other antimycotics for systemic use
J02AX01 - Flucytosine
Absorption
Rapidly and virtually completely absorbed following oral administration. Bioavailability 78% to 89%.
Route of Elimination
Flucytosine is excreted via the kidneys by means of glomerular filtration without significant tubular reabsorption. A small portion of the dose is excreted in the feces.
Flucytosine is rapidly and well absorbed from the GI tract, with plasma levels peaking in 1-2 hr in animals that have received the drug for several days. The drug is widely distributed in the body, with a volume of distribution approximating the total body water. Flucytosine is minimally bound to plasma proteins. There is excellent penetration into body fluids such as the CSF, synovial fluids, and aqueous humor.
Kahn, C.M (ed.).; The Merck Veterinary Manual 10th Edition. Merck & Co. Whitehouse Station NJ. 2010, p. 2310
Flucytosine is rapidly and almost completed absorbed from the GI tract. Bioavailability is 78-89% following oral administration. Food decreases the rate, but not the extent, of absorption.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 559
In a limited number of neonates receiving oral flucytosine in a dosage of 25, 50, or 100 mg/kg daily for the treatment of systemic candidiasis, median peak serum concentrations after 5 days of treatment were 19.6, 27.7, and 83.9 ug/mL, respectively, and the mean time to peak concentrations was 2.5 hours. There was considerable interindividual variation in serum concentrations, which did not correlate with gestational age, and some neonates had serum flucytosine concentrations greater than 100 ug/mL.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 559
In patients with normal renal function, peak serum flucytosine concentrations of 30-40 mcg/mL are reached within 2 hours following a single 2-g oral dose. In other studies in patients with normal renal function receiving a 6-week regimen of oral flucytosine (150 mg/kg daily given in divided doses every 6 hours) and concomitant IV amphotericin B, mean serum concentrations of flucytosine 1-2 hours after a dose were approximately 70-80 ug/mL.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 559
For more Absorption, Distribution and Excretion (Complete) data for Flucytosine (8 total), please visit the HSDB record page.
Flucytosine is deaminated, possibly by gut bacteria or by the fungal targets, to 5-fluorouracil, the active metabolite.
Flucytosine may be fungistatic or fungicidal in action depending on the concentration of the drug. Two possible mechanisms of action have been identified for flucytosine. Flucytosine appears to enter fungal cells via the action of fungal-specific cytosine permease. Inside the cell, flucytosine is converted into fluorouracil (5-FU) by cytosine deaminase and then after several intermediate steps is converted into 5-fluorouridine triphosphate (FUTP). FUTP is incorporated into fungal RNA and interferes with protein synthesis. Flucytosine also appears to be converted to 5-fluorodeoxyuridine monophosphate, which noncompetitively inhibits thymidylate synthetase and interferes with DNA synthesis. Flucytosine does not appear to have antineoplastic activity.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 559
The aim of this study is to investigate whether fluorouracil (5-FU) could be responsible for bone-marrow depression occurring in fluorocytosine (5-FC) treated patients. Six 5-FC treated patients were included in this pilot study. Toxicity was monitored by means of thrombocyte and leucocyte counts. 5-FC and 5-FU serum levels were measured using a high-performance liquid chromatography (HPLC) assay that allows simultaneous determination of both compounds. The amounts of 5-FU in the 34 available serum samples remained below the limit of quantitation (< 0.05 mg/L), whereas 5-FC levels could be detected in all samples. Instead, low levels of the 5-FU catabolite alpha-fluoro-beta-alanine (FBAL) were detected in several of the investigated serum samples. In case of three patients thrombocyte counts remained within the normal range during 5-FC treatment, whereas one patient developed thrombocytopenia (50 x 10(9) thrombocytes/L) during therapy. Furthermore, one patient developed leucocytopenia (2.6 x 10(9) leucocytes/L) during 5-FC therapy, whereas the remaining five patients were suffering from leucocytosis prior to 5-FC therapy. In conclusion, we found nondetectable 5-FU serum concentrations (< 0.05 mg/L) in ICU patients treated with intravenous 5-FC, making it unlikely that 5-FC-associated toxicity results from 5-FU exposure in patients receiving intravenous 5-FC therapy. These findings may be explained by the fact that our patients received 5-FC intravenously instead of orally, therefore not allowing active conversion of 5-FC to 5-FU by the human intestinal microflora. /5-Fluorouracil/
PMID:11903511 Vermes A et al; Fundam Clin Pharmacol 16 (1): 39-47 (2002)
A gas chromatographic-mass spectrometric method for detecting 5-fluorouracil (5-FU) in serum at concentrations as low as 10 ng/mL was used to determine to what extent 5-FU was present in the serum of patients taking oral 5-fluorocytosine (5-FC). Preliminary studies in two patients and two healthy volunteers given an initial 2-g oral dose of 5-FC demonstrated sustained serum 5-FU levels (>100 ng/mL) during the 5 hr after ingestion of drug. Pharmaceutical preparations of 5-FC used in these studies were shown to be insignificantly contaminated with 5-FU (<0.03%), suggesting in vivo conversion of 5-FC to 5-FU had occurred. Serum samples from seven patients with cryptococcal meningitis treated with amphotericin B and 5-FC were examined for 5-FU. Five of these patients had experienced hematological or other toxicity attributed to 5-FC at some time during the course of therapy. Of 41 serum samples, 20 were observed to have 5-FU levels greater than 1,000 ng/mL in the range observed with cancer chemotherapeutic doses of 5-FU known to be associated with hematological toxicity. It is concluded that conversion of 5-FC to 5-FU occurs in humans and furthermore that 5-FU may account for some of the toxicity observed with 5-FC. /5-Fluorouracil/
PMID:742878 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC352577 Diasio RB et al; Antimicrob Agents Chemother 14 (6): 903-8 (1978)
Metabolism of 5-fluorocytosine-6-14C (5-FC) was studied in mice, rats, rabbits and dogs after oral and subcutaneous, single and repeated administration. In the urines of all species, intact 5-FC accounted for more than 90% of the total radioactivity at any time of the various treatment schedules. The average proportion of the urinary metabolites was around 5% in dogs, 3% in rabbits, 2.5% in rats, and 2% in mice of the total radioactivity. At repeated dosage, there was an increase of metabolites in mice but a decrease in rats treated subcutaneously. Neither increase nor decrease was observed in rabbits (treated orally) and dogs. Two metabolites were identified, alpha-fluoro-beta-ureido-propionic acid (FUPA) and alpha-fluoro-beta-alanine, the latter occurring mainly after oral treatment. These compounds represent probably that part of 5-FC which was deaminated to 5-fluorouracil (5-FU) or directly to 5-fluorodihydrouracil. FUPA was the only metabolite found in the urines collected from 4 out of 5 human volunteers during the first 12 h after single oral administration of 3.5 g of the radiolabelled drug. Its maximum proportion was 1.1% of the total radioactivity. No metabolites were detected in the urine neither of the 5th volunteer nor in those of 3 mycosis patients who were given the radioactive dose after they had received regular chemotherapy with unlabelled 5-FC (150 mg/kg/day) for at least 2 weeks. The sensitivity threshold of the method was 0.1-0.4% of the total radioactivity. One of the patients had developed thrombocytopenia which was probably due to 5-FC chemotherapy. The symptoms of 5-FC intolerance were in most of the examined species similar to those observed with 5-FU [9]. However, no quantitative correlation between proportion of metabolites and 5-FC toxicity is apparent except that man is the species in which both metabolism and toxicity are the lowest. It has not been proved yet that 5-FC intolerance occurring in a small percentage of patients receiving 5-FC chemotherapy (mainly leukopenia, thrombocytopenia) results in fact from conversion to 5-FU.
PMID:773604 Polak A et al; Chemotherapy 22 (3-4): 137-53 (1976)
For more Metabolism/Metabolites (Complete) data for Flucytosine (6 total), please visit the HSDB record page.
2.4 to 4.8 hours.
In a limited number of infants, the median half-life of flucytosine was 7.4 hours.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 560
The half-life of flucytosine is prolonged in patients with renal insufficiency; the average half-life in nephrectomized or anuric patients was 85 hours (range: 29.9 to 250 hours). A linear correlation was found between the elimination rate constant of flucytosine and creatinine clearance.
NIH; DailyMed. Current Medication Information for Flucytosine Capsule (Updated: January 8, 2018). Available from, as of February 8, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58189ec7-118b-458a-8e4f-7867a97cfcbd
The elimination half-life of flucytosine has been variously reported to be 2.4-6 hours in patients with normal renal function, 6-14 hours in patients with creatinine clearances of 40 mL/minute, 12-15 hours in patients with creatinine clearances of 20 mL/minute, 21-27 hours in patients with creatinine clearances of 10 mL/minute, and 30-250 hours in patients with creatinine clearances less than 10 mL/minute. Half-lives up to 1160 hours have been reported in a few patients with creatinine clearances less than 2 mL/minute. Some clinicians have suggested that the half-life of flucytosine in hours is approximately 5 or 6 times the serum creatinine concentration in mg/dL.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 560
Although the exact mode of action is unknown, it has been proposed that flucytosine acts directly on fungal organisms by competitive inhibition of purine and pyrimidine uptake and indirectly by intracellular metabolism to 5-fluorouracil. Flucytosine enters the fungal cell via cytosine permease; thus, flucytosine is metabolized to 5-fluorouracil within fungal organisms. The 5-fluorouracil is extensively incorporated into fungal RNA and inhibits synthesis of both DNA and RNA. The result is unbalanced growth and death of the fungal organism. It also appears to be an inhibitor of fungal thymidylate synthase.
Flucytosine may be fungistatic or fungicidal in action depending on the concentration of the drug. Two possible mechanisms of action have been identified for flucytosine. Flucytosine appears to enter fungal cells via the action of fungal-specific cytosine permease. Inside the cell, flucytosine is converted into fluorouracil (5-FU) by cytosine deaminase and then after several intermediate steps is converted into 5-fluorouridine triphosphate (FUTP). FUTP is incorporated into fungal RNA and interferes with protein synthesis. Flucytosine also appears to be converted to 5-fluorodeoxyuridine monophosphate, which noncompetitively inhibits thymidylate synthetase and interferes with DNA synthesis. Flucytosine does not appear to have antineoplastic activity.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 559

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
39
PharmaCompass offers a list of Fluocytosine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fluocytosine manufacturer or Fluocytosine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fluocytosine manufacturer or Fluocytosine supplier.
PharmaCompass also assists you with knowing the Fluocytosine API Price utilized in the formulation of products. Fluocytosine API Price is not always fixed or binding as the Fluocytosine Price is obtained through a variety of data sources. The Fluocytosine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fluocytosine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fluocytosine, including repackagers and relabelers. The FDA regulates Fluocytosine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fluocytosine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fluocytosine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fluocytosine supplier is an individual or a company that provides Fluocytosine active pharmaceutical ingredient (API) or Fluocytosine finished formulations upon request. The Fluocytosine suppliers may include Fluocytosine API manufacturers, exporters, distributors and traders.
click here to find a list of Fluocytosine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fluocytosine DMF (Drug Master File) is a document detailing the whole manufacturing process of Fluocytosine active pharmaceutical ingredient (API) in detail. Different forms of Fluocytosine DMFs exist exist since differing nations have different regulations, such as Fluocytosine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fluocytosine DMF submitted to regulatory agencies in the US is known as a USDMF. Fluocytosine USDMF includes data on Fluocytosine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fluocytosine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fluocytosine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Fluocytosine Drug Master File in Japan (Fluocytosine JDMF) empowers Fluocytosine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Fluocytosine JDMF during the approval evaluation for pharmaceutical products. At the time of Fluocytosine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Fluocytosine suppliers with JDMF on PharmaCompass.
A Fluocytosine CEP of the European Pharmacopoeia monograph is often referred to as a Fluocytosine Certificate of Suitability (COS). The purpose of a Fluocytosine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Fluocytosine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Fluocytosine to their clients by showing that a Fluocytosine CEP has been issued for it. The manufacturer submits a Fluocytosine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Fluocytosine CEP holder for the record. Additionally, the data presented in the Fluocytosine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Fluocytosine DMF.
A Fluocytosine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Fluocytosine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Fluocytosine suppliers with CEP (COS) on PharmaCompass.
A Fluocytosine written confirmation (Fluocytosine WC) is an official document issued by a regulatory agency to a Fluocytosine manufacturer, verifying that the manufacturing facility of a Fluocytosine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Fluocytosine APIs or Fluocytosine finished pharmaceutical products to another nation, regulatory agencies frequently require a Fluocytosine WC (written confirmation) as part of the regulatory process.
click here to find a list of Fluocytosine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Fluocytosine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Fluocytosine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Fluocytosine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Fluocytosine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Fluocytosine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Fluocytosine suppliers with NDC on PharmaCompass.
Fluocytosine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fluocytosine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fluocytosine GMP manufacturer or Fluocytosine GMP API supplier for your needs.
A Fluocytosine CoA (Certificate of Analysis) is a formal document that attests to Fluocytosine's compliance with Fluocytosine specifications and serves as a tool for batch-level quality control.
Fluocytosine CoA mostly includes findings from lab analyses of a specific batch. For each Fluocytosine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fluocytosine may be tested according to a variety of international standards, such as European Pharmacopoeia (Fluocytosine EP), Fluocytosine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fluocytosine USP).
