
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
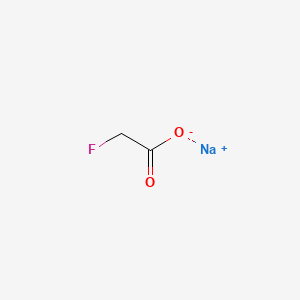

1. Compound 1080
2. Fluoroacetic Acid
3. Fluoroacetic Acid, 18f-labeled
4. Fluoroacetic Acid, Aluminum Salt
5. Fluoroacetic Acid, Ammonium Salt
6. Fluoroacetic Acid, Ammonium Salt, 2-(14)c-labeled
7. Fluoroacetic Acid, Barium Salt
8. Fluoroacetic Acid, Cadmium Salt
9. Fluoroacetic Acid, Calcium Salt
10. Fluoroacetic Acid, Copper (2+) Salt
11. Fluoroacetic Acid, Lead (+4) Salt
12. Fluoroacetic Acid, Magnesium Salt
13. Fluoroacetic Acid, Mercury (2+) Salt
14. Fluoroacetic Acid, Potassium Salt
15. Fluoroacetic Acid, Sodium Salt
16. Fluoroacetic Acid, Terbium (+3) Salt
17. Monofluoroacetate
18. Monofluoroacetic Acid
19. Sodium (18f)fluoroacetate
1. 1080
2. Sodium Monofluoroacetate
3. 62-74-8
4. Compound 1080
5. Natriumfluoracetat
6. Furatol
7. Yasoknock
8. Fratol
9. Sodium Fluoroacetate [iso]
10. Fluoroacetic Acid Sodium Salt
11. Sodium;2-fluoroacetate
12. Sodium Fluoacetate
13. Sodium Fluoracetate
14. Sodium Fluoacetic Acid
15. Ratbane 1080
16. Sodium 2-fluoroacetate
17. Natriumfluoracetaat
18. Fluoroacetic Acid Sodium
19. Fluoroacetic Acid, Sodium Salt
20. Sodio, Fluoracetato Di
21. Sodium Fluoroacetate De
22. Chebi:38699
23. Monofluoressigsaures Natrium
24. 166wtm3843
25. Tenate
26. Fluorakil 3
27. Caswell No. 770
28. Fluoroctan Sodny
29. Fluoroctan Sodny [czech]
30. Latka 1080 [czech]
31. Natriumfluoracetaat [dutch]
32. Natriumfluoracetat [german]
33. Rcra Waste Number P058
34. Hsdb 743
35. Nsc-77690
36. Sodium Fluoroacetate De [french]
37. Fluoroacetate De Sodium
38. Sodio, Fluoracetato Di [italian]
39. Acetic Acid, Fluoro-, Sodium Salt
40. Latka 1080
41. Einecs 200-548-2
42. Fluoroacetic Acid Sodium Salt [bsi]
43. Monofluoressigsaures Natrium [german]
44. Nsc 77690
45. Un2629
46. Fluoroacetate De Sodium [iso-french]
47. Rcra Waste No. P058
48. Epa Pesticide Chemical Code 075003
49. Ai3-08434
50. Unii-166wtm3843
51. Sodium Fluoro-acetate
52. Spectrum2_000664
53. Spectrum3_001693
54. Spectrum4_000925
55. Spectrum5_001848
56. Dsstox_cid_4311
57. Dsstox_rid_77363
58. Dsstox_gsid_24311
59. Kbiogr_001450
60. Spectrum330009
61. Schembl226060
62. Spbio_000727
63. Chembl369611
64. Dtxsid8024311
65. Kbio3_002466
66. Sodium Fluoroacetate [hsdb]
67. Tox21_301420
68. Ccg-40063
69. Mfcd00002682
70. Sodium Fluoroacetate [mart.]
71. Akos025295568
72. Cas-62-74-8
73. Ncgc00095771-01
74. Ncgc00255312-01
75. Fluoroacetic Acid Sodium Salt [mi]
76. Sodium Fluoroacetate [un2629] [poison]
77. F0031
78. Acetic Acid, 2-fluoro-, Sodium Salt (1:1)
79. C18588
80. Q410911
81. Fluoroacetic Acid Sodium 10 Microg/ml In Water. Short Expiry Date Due To Chemical Nature Of Component(s)
| Molecular Weight | 100.02 g/mol |
|---|---|
| Molecular Formula | C2H2FNaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 99.99365175 g/mol |
| Monoisotopic Mass | 99.99365175 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 46.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
The estimated lethal dose in humans ranges from 2 to 10 mg/kg. /Fluoroacetate/
Klaassen, C.D. (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001., p. 801
Rodenticides
Substances used to destroy or inhibit the action of rats, mice, or other rodents. (See all compounds classified as Rodenticides.)
... Rapidly absorbed by GI tract. It is not well absorbed by intact skin, but absorption may be greater in presence of dermatitis or other skin injury.
Krieger, R. (ed.). Handbook of Pesticide Toxicology. Volume 2, 2nd ed. 2001. Academic Press, San Diego, California., p. 1795
... Samples taken at autopsy from man who survived about 17 hours after being found unconscious ... /showed concn in/ urine, 368 ppm; liver, 58 ppm; brain, 76 ppm; and kidney, 65 ppm.
Krieger, R. (ed.). Handbook of Pesticide Toxicology. Volume 2, 2nd ed. 2001. Academic Press, San Diego, California., p. 1798
...Liver of person whose death was supposed due to sodium fluoroacetate contained 2.4 mg of fluorine/100 g.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 178
Amount of sodium monofluoroacetate recovered from brain per unit weight of tissue was about 2 times that from other organs after rabbits were killed with dose 10 times LD50. Amount of organofluorine was about the same 24 hr after death as immediately after death and decreased gradually, by 10-20% after 3 days and by 50-80% when carcass was kept at 21-25 C.
Tomiya Y et al; Nippon Hoigaku Zasshi (Jpn J Leg Med) 28 (6): 461 (1975)
For more Absorption, Distribution and Excretion (Complete) data for SODIUM FLUOROACETATE (8 total), please visit the HSDB record page.
Following injection of fluoroacetate-2-(14)C into rats, about 3% of label appeared in respiratory carbon dioxide and 32% in urine within 4 days. Labeled fluorocitrate was recovered from urine. Incorporation of label into cholesterol and into... lower and higher fatty acid fractions was demonstrated. Other unidentified labeled materials /were/... observed.
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 211
When fluoroacetate-2-(14)C was applied to plants (acacia geoginae, castor bean, peanut, pinto bean), labeled carbon monoxide was observed. Some label... was also incorporated into water soluble fractions and lipids. In some plant seeds... /it/ was converted into fluorine-containing long chain fatty acids.
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 211
Sodium fluoroacetate is converted into fluorocitric acid in body and blocks TCA cycle by inhibiting aconitase activity. Poisoning can be diagnosed by increased citric acid levels in all organs or by determination of aconitase activity.
Gruender HD; Deut Tieraerztl Wochenschr 80 (19): 451-8 (1973)
2,4-Dinitrofluorobenzene reacts with glutathione to form a stable product similar to that formed with the model glutathione-S-transferase substrate, 1-chloro-2,4-dinitrobenzene. ... Fluoroacetamide, like fluoroacetate, undergoes no discernable chemical defluorination. Its enzymatic defluorination is approx 10% of that observed for fluoroacetate and only 0.2% of the rate for 2,4-dinitrofluorobenzene. An antibody raised to the fluoroacetate specific dehalogenase precipitated both fluoroacetate ad fluoroacetamide defluorinating activity but had no effect on either 1-chloro-2,4-dinitrobenzene or 2,4-dinitrofluorobenzene activity. ... 2,4-Dinitrofluorobenzene is metabolized by the glutathione-S-transferase while fluoroacetamide is metabolized by the fluoroacetate specific dehalogenase.
Kostyniak PJ, Soiefer AI; Toxicol Let 22 (920): 217-22 (1984)
For more Metabolism/Metabolites (Complete) data for SODIUM FLUOROACETATE (7 total), please visit the HSDB record page.
Plasma elimination half-life in rabbits was shown to be 1.1 hour and the retention time in tissue greater with larger doses. Tissue residues were substantially lower than in plasma.
Krieger, R. (ed.). Handbook of Pesticide Toxicology. Volume 2, 2nd ed. 2001. Academic Press, San Diego, California., p. 1795
Sodium monofluoroacetate (1080) was administered orally to sheep and goats at a dose level of 0.1 mg/kg bw. The plasma elimination half-life was 10.8 hr in sheep and 5.4 hr in goats.
PMID:7908808 Eason C et al; Hum Exp Toxicol 13 (2): 119-22 (1994)
Sodium fluoroacetate (SFAC) or Compound 1080 is a potent rodenticide, largely used after 1946 for rodent and home pest control. The toxic effects of SFAC are caused by fluorocitrate action, a toxic metabolite, which has a competitive action with aconitase enzyme, leading to citrate accumulation and resulting in interference in energy production by Krebs cycle blockade. ...
PMID:16696292 Collicchio-Zuanaze RC et al; Hum Exp Toxicol. 25(4):175-82 (2006).
Sodium monofluoroacetate (1080) is a vertebrate pesticide widely used for possum control in New Zealand. Fluoroacetate is also a toxic component of poisonous plants found in Australia, South Africa, South America, and India. Because of its importance and effectiveness in pest control and the highly toxic nature of this compound, its acute sub-lethal and target organ toxicity have been extensively studied. In relation to its use as a pesticide its environmental fate, persistence, non-target impacts and general toxicology have been and continue to be extensively studied. Toxic baits must be prepared and used with extreme care, otherwise humans, livestock, and non-target wildlife will be put at risk. The high risk of secondary poisoning of dogs is a cause for concern. 1080 acts by interfering with cellular energy production. Possums die from heart failure, usually within 6-18 hr of eating baits. Long-term exposure to sub-lethal doses can have harmful effects and strict safety precautions are enforced to protect contractors and workers in the bait manufacturing industry. Considerable care is taken when using 1080 to ensure that the risks of using it are outweighed by the ecological benefits achieved from its use. When its use is controversial, risk communicators must take care not to trivialize the toxicity of the compound. The benefits of 1080 use in conservation, pest control, and disease control should be weighed up alongside the risks of using 1080 and other techniques for pest control.
PMID:12505362 Eason C; Toxicology. 181-182:523-30 (2002).
Active neurons have a high demand for energy substrate, which is thought to be mainly supplied as lactate by astrocytes. Heavy lactate dependence of neuronal activity suggests that there may be a mechanism that detects and controls lactate levels and/or gates brain activation accordingly. Here, we demonstrate that orexin neurons can behave as such lactate sensors. Using acute brain slice preparations and patch-clamp techniques, we show that the monocarboxylate transporter blocker alpha-cyano-4-hydroxycinnamate (4-CIN) inhibits the spontaneous activity of orexin neurons despite the presence of extracellular glucose. Furthermore, fluoroacetate, a glial toxin, inhibits orexin neurons in the presence of glucose but not lactate. Thus, orexin neurons specifically use astrocyte-derived lactate. The effect of lactate on firing activity is concentration dependent, an essential characteristic of lactate sensors. Furthermore, lactate disinhibits and sensitizes these neurons for subsequent excitation. 4-CIN has no effect on the activity of some arcuate neurons, indicating that lactate dependency is not universal. Orexin neurons show an indirect concentration-dependent sensitivity to glucose below 1 mm, responding by hyperpolarization, which is mediated by ATP-sensitive potassium channels composed of Kir6.1 and SUR1 subunits. In conclusion, our study suggests that lactate is a critical energy substrate and a regulator of the orexin system. Together with the known effects of orexins in inducing arousal, food intake, and hepatic glucose production, as well as lactate release from astrocytes in response to neuronal activity, our study suggests that orexin neurons play an integral part in balancing brain activity and energy supply.
PMID:20554857 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6634592 Parsons MP and Hirasawa M; J Neurosci. 30(24):8061-70 (2010).
Sodium fluoroacetate was introduced as a rodenticide in the US in 1946. However, its considerable efficacy against target species is offset by comparable toxicity to other mammals and, to a lesser extent, birds and its use as a general rodenticide was therefore severely curtailed by 1990. Currently, sodium fluoroacetate is licensed in the US for use against coyotes, which prey on sheep and goats, and in Australia and New Zealand to kill unwanted introduced species. The extreme toxicity of fluoroacetate to mammals and insects stems from its similarity to acetate, which has a pivotal role in cellular metabolism. Fluoroacetate combines with coenzyme A (CoA-SH) to form fluoroacetyl CoA, which can substitute for acetyl CoA in the tricarboxylic acid cycle and reacts with citrate synthase to produce fluorocitrate, a metabolite of which then binds very tightly to aconitase, thereby halting the cycle. Many of the features of fluoroacetate poisoning are, therefore, largely direct and indirect consequences of impaired oxidative metabolism. Energy production is reduced and intermediates of the tricarboxylic acid cycle subsequent to citrate are depleted. Among these is oxoglutarate, a precursor of glutamate, which is not only an excitatory neurotransmitter in the CNS but is also required for efficient removal of ammonia via the urea cycle. Increased ammonia concentrations may contribute to the incidence of seizures. Glutamate is also required for glutamine synthesis and glutamine depletion has been observed in the brain of fluoroacetate-poisoned rodents. Reduced cellular oxidative metabolism contributes to a lactic acidosis. Inability to oxidise fatty acids via the tricarboxylic acid cycle leads to ketone body accumulation and worsening acidosis. Adenosine triphosphate (ATP) depletion results in inhibition of high energy-consuming reactions such as gluconeogenesis. Fluoroacetate poisoning is associated with citrate accumulation in several tissues, including the brain. Fluoride liberated from fluoroacetate, citrate and fluorocitrate are calcium chelators and there are both animal and clinical data to support hypocalcaemia as a mechanism of fluoroacetate toxicity. However, the available evidence suggests the fluoride component does not contribute. Acute poisoning with sodium fluoroacetate is uncommon. Ingestion is the major route by which poisoning occurs. Nausea, vomiting and abdominal pain are common within 1 hour of ingestion. Sweating, apprehension, confusion and agitation follow. Both supraventricular and ventricular arrhythmias have been reported and nonspecific ST- and T-wave changes are common, the QTc may be prolonged and hypotension may develop. Seizures are the main neurological feature. Coma may persist for several days. Although several possible antidotes have been investigated, they are of unproven value in humans. The immediate, and probably only, management of fluoroacetate poisoning is therefore supportive, including the correction of hypocalcaemia.
PMID:17288493 Proudfoot AT et al; Toxicol Rev. 25(4):213-9 (2006).
For more Mechanism of Action (Complete) data for SODIUM FLUOROACETATE (7 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
ABOUT THIS PAGE
84
PharmaCompass offers a list of Fluoroacetic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fluoroacetic Acid manufacturer or Fluoroacetic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fluoroacetic Acid manufacturer or Fluoroacetic Acid supplier.
PharmaCompass also assists you with knowing the Fluoroacetic Acid API Price utilized in the formulation of products. Fluoroacetic Acid API Price is not always fixed or binding as the Fluoroacetic Acid Price is obtained through a variety of data sources. The Fluoroacetic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fluoroacetic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fluoroacetic Acid, including repackagers and relabelers. The FDA regulates Fluoroacetic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fluoroacetic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Fluoroacetic Acid supplier is an individual or a company that provides Fluoroacetic Acid active pharmaceutical ingredient (API) or Fluoroacetic Acid finished formulations upon request. The Fluoroacetic Acid suppliers may include Fluoroacetic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Fluoroacetic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fluoroacetic Acid DMF (Drug Master File) is a document detailing the whole manufacturing process of Fluoroacetic Acid active pharmaceutical ingredient (API) in detail. Different forms of Fluoroacetic Acid DMFs exist exist since differing nations have different regulations, such as Fluoroacetic Acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fluoroacetic Acid DMF submitted to regulatory agencies in the US is known as a USDMF. Fluoroacetic Acid USDMF includes data on Fluoroacetic Acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fluoroacetic Acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fluoroacetic Acid suppliers with USDMF on PharmaCompass.
Fluoroacetic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fluoroacetic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fluoroacetic Acid GMP manufacturer or Fluoroacetic Acid GMP API supplier for your needs.
A Fluoroacetic Acid CoA (Certificate of Analysis) is a formal document that attests to Fluoroacetic Acid's compliance with Fluoroacetic Acid specifications and serves as a tool for batch-level quality control.
Fluoroacetic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Fluoroacetic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fluoroacetic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Fluoroacetic Acid EP), Fluoroacetic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fluoroacetic Acid USP).

