



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
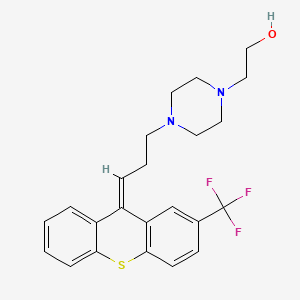

1. Alpha-flupenthixol
2. Cis-flupenthixol
3. Emergil
4. Fluanxol
5. Flupenthixol
1. Cis-flupentixol
2. Fluanxol
3. Cis-(z)-flupenthixol
4. Flupenthixol
5. Fluxanxol
6. Emergil
7. Cis-flupenthixol
8. Siplaril
9. Siplarol
10. Alpha-flupenthixol
11. Zuflupentixol
12. 53772-82-0
13. Lc 44
14. Flupenthixole
15. Fa0uyh6quo
16. 2709-56-0
17. Fluphenthixol
18. 2-[4-[(3z)-3-[2-(trifluoromethyl)thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethanol
19. Cis-(z)-flupenthixol Dihydrochloride
20. Chebi:10454
21. N 7009
22. N-7009
23. 4-(3-(2-(trifluoromethyl)-9h-thioxanthen-9-ylidene)propyl)-1-piperazineethanol
24. 4-(3-(2-(trifluoromethyl)thioxanthen-9-ylidene)propyl)-1-piperazineethanol
25. 1-piperazineethanol, 4-(3-(2-(trifluoromethyl)-9h-thioxanthen-9-ylidene)propyl)-
26. 1-piperazineethanol, 4-(3-(2-(trifluoromethyl)-9h-thioxanthen-9-ylidene)propyl)-, (z)-
27. Ncgc00162179-02
28. 1-piperazineethanol, 4-(3-(2-(trifluoromethyl)thioxanthen-9-ylidene)propyl)-
29. Dsstox_cid_26310
30. Dsstox_rid_81531
31. Dsstox_gsid_46310
32. Flupentiol
33. Mls001332581
34. Flupentixolum [inn-latin]
35. (z)-4-(3-(2-(trifluoromethyl)-9h-thioxanthen-9-ylidene)propyl)piperazine-1-ethanol
36. (z)-4-[3-[2-(trifluoromethyl)-9h-thioxanthen-9-ylidene]propyl]piperazine-1-ethanol
37. 1-piperazineethanol, 4-[3-[2-(trifluoromethyl)-9h-thioxanthen-9-ylidene]propyl]-
38. 2-trifluoromethyl-9-(3-(4-(2-hydroxyethyl)piperazin-1-yl)propylidene)thioxanthene
39. 4-[3-[2-(trifluoromethyl)-9h-thioxanthen-9-ylidene]propyl]-1-piperazineethanol
40. Cas-53772-82-0
41. Unii-fa0uyh6quo
42. Smr000875208
43. Einecs 220-304-9
44. Einecs 258-756-4
45. Flupentixolo
46. Flurentixol
47. Flupentixol [inn:ban:dcf]
48. Cis Flupenthixol
49. (z)-flupentixol
50. 1-piperazineethanol, 4-[(3z)-3-[2-(trifluoromethyl)-9h-thioxanthen-9-ylidene]propyl]-
51. 2-trifluoromethyl-9-[3-[4-(2-hydroxyethyl)piperazin-1-yl]propylidene]thioxanthene
52. Cis(z)flupenthixol
53. 1-piperazineethanol, 4-((3z)-3-(2-(trifluoromethyl)-9h-thioxanthen-9-ylidene)propyl)-
54. Depixol (tn)
55. Flupentixol (inn)
56. Z-flupenthixol
57. Z-flupentixol
58. Prestwick2_000340
59. Prestwick3_000340
60. Biomol-nt_000021
61. Flupentixol, (z)-
62. (z)-4-(3-(2-(trifluoromethyl)-9h-thioxanthen-9-ylidene)propyl)-1-piperazineethanol
63. .alpha.-flupenthixol
64. Unii-21hmq851is
65. Lopac0_000528
66. Schembl34200
67. Bspbio_000379
68. Gtpl948
69. Chembl54661
70. 21hmq851is
71. Bpbio1_000417
72. Bpbio1_001183
73. Dtxsid9046310
74. Bdbm79172
75. (z)-2-[4-[3-[2-(trifluoromethyl)thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethanol
76. Cid_10140115
77. Lc-44
78. Tox21_112003
79. Zinc29489118
80. Tox21_112003_1
81. Ccg-204618
82. Db00875
83. Sdccgsbi-0050511.p002
84. Ncgc00162179-01
85. Ncgc00162179-03
86. Ncgc00162179-04
87. Ncgc00162179-05
88. Ncgc00162179-06
89. Ncgc00162179-08
90. Ncgc00162179-12
91. D01044
92. L000972
93. Q420350
94. W-107130
95. Brd-k70487031-001-01-0
96. (z)-2-(4-(3-(2-(trifluoromethyl)-9h-thioxanthen-9-ylidene)propyl)piperazin-1-yl)ethanol
97. 2-(4-(3-[2-(trifluoromethyl)-9h-thioxanthen-9-ylidene]propyl)-1-piperazinyl)ethanol #
98. 2-trifluoromethyl-9-(3-(4-(.beta.-hydroxyethyl)-1-piperazinyl)propylidene)thioxanthene
99. 2-[4-[(3z)-3-[2-(trifluoromethyl)-9-thioxanthenylidene]propyl]-1-piperazinyl]ethanol;hydrochloride
100. 2-[4-[(3z)-3-[2-(trifluoromethyl)thioxanthen-9-ylidene]propyl]piperazino]ethanol;hydrochloride
101. 4-((3z)-3-(2-(trifluoromethyl)-9h-thioxanthen-9-ylidene)propyl)-1-piperazineethanol
| Molecular Weight | 434.5 g/mol |
|---|---|
| Molecular Formula | C23H25F3N2OS |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 434.16396908 g/mol |
| Monoisotopic Mass | 434.16396908 g/mol |
| Topological Polar Surface Area | 52 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 592 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Flupentixol is indicated for maintenance therapy of chronic schizophrenic patients whose main manifestations do not include excitement, agitation or hyperactivity. It is indicated for the management of depression in adult patients who may, or may not, also be showing signs of anxiety. Flupentixol in combination with [melitracen] is indicated to manage symptoms of anxiety, depression, and asthenia in adults.
Flupentixol is an antipsychotic agent with anxiolytic and mild sedative actions. It exerts weak anticholinergic and adrenergic effects. It possesses antiemetic actions. As flupentixol works by antagonizing dopamine actions, it can cause extrapyramidal effects, mostly at doses greater than 10 mg. In clinical trials, flupentixol-induced extrapyramidal effects have been managed with anti-Parkinsonian drugs. Drug esterification in the intramuscular formulation of the drug results in slow release of the drug from the injection site and a prolonged duration of action. Flupentixol has been investigated for use in mild to moderate depression: compared to other antidepressant agents, flupentixol has a rapid onset of action, where antidepressive effects were observed within the first two to three days after administration. As with other antipsychotic agents, flupentixol can cause QTc prolongation and increase the risk of arrhythmias. In clinical trials, flupentixol was associated with the risk of cardiovascular disease, cerebrovascular adverse events, stroke, and venous thromboembolism. Flupentixol can elevate the levels of prolactin; however, the clinical significance of hyperprolactinemia caused by neuroleptic drugs is unclear. Long-term hyperprolactinemia, when associated with hypogonadism, may lead to decreased bone mineral density in both female and male subjects. Interestingly, recent studies show that flupentixol exhibits anti-tumour properties alone or synergistically with other anticancer drugs like gefitinib. One study demonstrated that _in vitro_, flupentixol docks to the ATP binding pocket of phosphatidylinositol 3-kinase (PI3K), a lipid kinase that activates signalling pathways that are often hyperactivated in some cancers. Flupentixol inhibited the PI3K/AKT pathway and survival of lung cancer cells _in vitro_ and _in vivo_.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AF - Thioxanthene derivatives
N05AF01 - Flupentixol
Absorption
Following oral administration, flupentixol is readily absorbed from the gastrointestinal tract, with oral bioavailability of about 40%. Tmax ranges from three to eight hours. Steady-state plasma levels are achieved in about seven days and following once-daily oral administration of 5 mg flupentixol, the mean minimum steady-state level was about 1.7 ng/mL (3.9 nmol/L). From the site of intramuscular injection, esterified flupentixol diffuses slowly from the oil solution and is slowly released into the extracellular fluid and the circulation to be distributed to different tissues. Peak drug concentrations are reached between four and seven days following intramuscular injection. Intramuscularly administered flupentixol is detectable in the blood three weeks after injection and reaches steady-state concentrations after about three months of repeated administration.
Route of Elimination
Fecal excretion is more predominant than renal excretion. In the feces, flupentixol is recovered in the feces mainly as the unchanged form, as well as its lipophilic metabolites, such as dealkyl-flupentixol. Flupentixol is recovered in the urine as the unchanged form as well as its hydrophilic sulfoxide and glucuronide metabolites.
Volume of Distribution
The apparent volume of distribution is about 14.1 L/kg. Following administration, the highest levels of flupentixol are found in the lungs, liver, and spleen. Lower concentrations of the drug are found in the blood and brain.
Clearance
Following oral administration, the mean systemic clearance is about 0.29 L/min.
Flupentixol is metabolized in the liver via sulfoxidation, dealkylation, and glucuronidation to form pharmacologically inactive metabolites. Flupentixol decanoate, the active ingredient in the intramuscular formulation, is hydrolyzed to flupentixol.
The elimination half-life is about 35 hours following oral administration and three weeks following intramuscular administration.
The mechanism of action of flupentixol is not completely understood. The antipsychotic actions are mainly thought to arise from cis(Z)-flupentixol, the active stereoisomer, acting as an antagonist at both dopamine D1 and D2 receptors with equal affinities. Schizophrenia is a mental illness characterized by positive (such as hallucinations and delusions) and negative (such as affect flattening and apathy) symptoms. While several neurotransmitter systems are implicated in the pathophysiologic processes leading to the development of symptoms, the dopamine and glutamate systems have been extensively studied. It is generally understood that positive symptoms of schizophrenia arise from a dysregulated striatal dopamine pathway, leading to hyperstimulation of D2 receptors. Many antipsychotic agents work by blocking D2 receptors as antagonists; similarly, cis(Z)-flupentixol, the active stereoisomer, is an antagonist at D2 receptors. However, there is now evidence that antipsychotic agents can work by blocking other dopamine receptor subtypes, such as D1, D3, or D4 receptors. One study showed that cis(Z)-flupentixol is an antagonist at both dopamine D1 and D2 receptors with equal affinities, and binds to D3 and D4 receptors with lower affinities. It also binds to alpha-1 adrenoceptors. Antidepressant effects of flupentixol are understood to be mediated by antagonism at 5-HT2A receptors, which are commonly downregulated following repeated antidepressant treatment. Flupentixol also binds to 5-HT2C receptors.


