
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
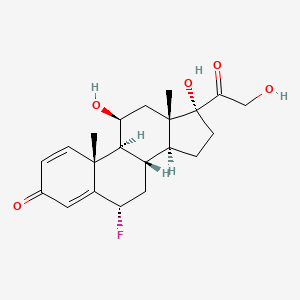

1. 53-34-9
2. Alphadrol
3. Etadrol
4. Isopredon
5. Vladicort
6. 6alpha-fluoroprednisolone
7. F. I. 6150
8. Nsc 47439
9. Nsc-47439
10. 6
11. A-fluoroprednisolone
12. Pregna-1,4-diene-3,20-dione, 6-fluoro-11,17,21-trihydroxy-, (6alpha,11beta)-
13. U 7800
14. U-7800
15. 6-alpha-fluoroprednisolone
16. (6s,8s,9s,10r,11s,13s,14s,17r)-6-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthren-3-one
17. Chebi:34474
18. Nsc47439
19. 9h05937g3x
20. Ncgc00182067-02
21. B 673
22. Dsstox_cid_26067
23. Dsstox_rid_81317
24. Dsstox_gsid_46067
25. Fluprednisolonum
26. Cas-53-34-9
27. Prednisolone, 6alpha-fluoro-
28. Fluprednisolonum [inn-latin]
29. Hsdb 3335
30. 6.alpha.-fluoroprednisolone
31. Einecs 200-170-8
32. 6alpha-fluoro-1-dehydrohydrocortisone
33. Prednisolone, 6.alpha.-fluoro-
34. Fi 6150
35. Corticosterone, 1-dehydro-6alpha-fluoro-
36. Unii-9h05937g3x
37. Pregna-1,4-diene-3,20-dione, 6-fluoro-11,17,21-trihydroxy-, (6.alpha.,11.beta.)-
38. Alphadrol (tn)
39. Ncgc00159474-02
40. Fluprednisolone [usan:inn:ban:nf]
41. Corticosterone, 1-dehydro-6.alpha.-fluoro-
42. 6alpha-fluprednisolone
43. 6alpha-fluoro-1,4-pregnadiene-11beta,17alpha,21-triol-3,20-dione
44. 6alpha-fluoro-11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione
45. Pregna-1,4-diene-3,20-dione, 6alpha-fluoro-11beta,17,21-trihydroxy-
46. Fluprednisolone [mi]
47. Fluprednisolone (usan/inn)
48. Schembl24232
49. 6- Alpha -fluoroprednisolone
50. Fluprednisolone [inn]
51. Fluprednisolone [hsdb]
52. Fluprednisolone [usan]
53. Fluprednisolone [mart.]
54. 6.alpha.-fluoro-11.beta.,17,21-trihydroxypregna-1,4-diene-3,20-dione
55. Chembl1200774
56. Dtxsid5046067
57. Fluprednisolone [who-dd]
58. Zinc3875362
59. Tox21_111698
60. Tox21_113143
61. Fluprednisolone [orange Book]
62. Nsc47439nsc 47439
63. Tox21_111698_1
64. Db09378
65. Ncgc00159474-03
66. Hy-107935
67. Cs-0030934
68. D04227
69. 053f349
70. Q732234
71. 6-alpha-fluoroprednisolone 100 Microg/ml In Acetonitrile
72. 6-alpha-fluoroprednisolone, Vetranal(tm), Analytical Standard
73. Pregna-1,20-dione, 6.alpha.-fluoro-11.beta.,17,21-trihydroxy-
74. 6.alpha.-fluoro-11.beta.,21-trihydroxypregna-1,4-diene-3,20-dione
75. 6alpha-fluoro-11beta,17alpha,21-trihydroxypregna-1,4-diene-3,20-dione
76. Pregna-1,20-dione, 6-fluoro-11,17,21-trihydroxy-, (6.alpha.,11.beta.)-
| Molecular Weight | 378.4 g/mol |
|---|---|
| Molecular Formula | C21H27FO5 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 378.18425212 g/mol |
| Monoisotopic Mass | 378.18425212 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 760 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Steroidal; Glucocorticoids, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
.../FLUPREDNISOLONE/ IS APPROX 2.5 TIMES AS POTENT AS PREDNISOLONE & 40 TIMES AS POTENT AS CORTISONE. HOWEVER, OCCASIONALLY IT DOES NOT APPEAR...ABLE TO CONTROL ALLERGIC OR INFLAMMATORY CONDITIONS THAT CAN BE CONTROLLED WITH OTHER GLUCOCORTICOIDS. FLUPREDNISOLONE ALSO HAS ERRATIC MINERALOCORTICOID ACTIVITY.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 898
...USEFUL IN TREATING INFLAMMATORY & ALLERGIC CONDITIONS & OTHER DISEASES THAT RESPOND TO GLUCOCORTICOIDS. IN ANTI-INFLAMMATORY EFFECT, 1.5 MG FLUPREDNISOLONE IS EQUIVALENT TO 20 MG HYDROCORTISONE.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 519
MEDICATION (VET): Glucocorticoids have profound effects on nearly all cell types and organ systems, particularly immunologic and inflammatory activity. They may be used in either an anti-inflammatory or immunosuppressive capacity, depending on the dosage selected. Glucocorticoids are used for hypersensitivity dermatoses, contact dermatitis, immune-mediated diseases (eg, pemphigus, pemphigoid, lupus erythematosus), and neoplasia (eg, mast cell tumor, lymphoma). ... They may be administered PO, IV, IM, or SC. /Glucocorticoids/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2008
SINCE ITS MINERALOCORTICOID EFFECTS ARE POORLY DEFINED, FLUPREDNISOLONE IS NOT RECOMMENDED FOR REPLACEMENT THERAPY IN ADRENOCORTICAL INSUFFICIENCY.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 520
HYDROCORTISONE MAY INCR RENAL EXCRETION OF ASPIRIN & RESULT IN DECR SALICYLATE LEVELS DURING CONCURRENT THERAPY. ... ASPIRIN & HYDROCORTISONE MAY THEORETICALLY EXERT COMBINED DELETERIOUS EFFECTS ON GASTRIC MUCOSA... /HYDROCORTISONE/
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 18
HYPERGLYCEMIC ACTION OF CORTISONE MAY OFFSET HYPOGLYCEMIC EFFECT OF CHLORPROPAMIDE... THERE IS POSSIBILITY THAT CONCURRENT ADMIN OF CHLORPROPAMIDE & CORTISONE MAY INCR LIKELIHOOD OF PPT GASTRIC ULCER, BUT THERE ARE NO CLINICAL REPORTS OF THIS...IN HUMANS. /CORTISONE/
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 38
The immunosuppressive effects of glucocorticoids may result in activation of latent infection or exacerbation of intercurrent infections, including those caused by Candida, Mycobacterium, Toxoplasma, Strongyloides, Pneumocystis, Cryptococcus, Nocardia, or Ameba. Glucocorticoids should be used with great care in patients with known or suspected Strongyloides (threadworm) infection. In such patients, glucocorticoid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia. /Corticosteroids/
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3033
For more Drug Warnings (Complete) data for FLUPREDNISOLONE (29 total), please visit the HSDB record page.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
EXCELLENT CORRELATIONS WERE OBTAINED BETWEEN DISSOLUTION RATES OF SOLVATED & NON-SOLVATED PHASES OF FLUPREDNISOLONE & IN VIVO DISSOLUTION RATES OF PELLET IMPLANTS IN RATS.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 423
CONJUGATED MOSTLY IN LIVER BUT ALSO IN KIDNEY /HUMAN, ORAL/.
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1963
Glucocorticoids are capable of suppressing the inflammatory process through numerous pathways. They interact with specific intracellular receptor proteins in target tissues to alter the expression of corticosteroid-responsive genes. Glucocorticoid-specific receptors in the cell cytoplasm bind with steroid ligands to form hormone-receptor complexes that eventually translocate to the cell nucleus. There these complexes bind to specific DNA sequences and alter their expression. The complexes may induce the transcription of mRNA leading to synthesis of new proteins. Such proteins include lipocortin, a protein known to inhibit PLA2a and thereby block the synthesis of prostaglandins, leukotrienes, and PAF. Glucocorticoids also inhibit the production of other mediators including AA metabolites such as COX, cytokines, the interleukins, adhesion molecules, and enzymes such as collagenase. /Glucocorticoids/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2128
ABOUT THIS PAGE
65
PharmaCompass offers a list of Fluprednisolone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fluprednisolone manufacturer or Fluprednisolone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fluprednisolone manufacturer or Fluprednisolone supplier.
PharmaCompass also assists you with knowing the Fluprednisolone API Price utilized in the formulation of products. Fluprednisolone API Price is not always fixed or binding as the Fluprednisolone Price is obtained through a variety of data sources. The Fluprednisolone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fluprednisolone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fluprednisolone, including repackagers and relabelers. The FDA regulates Fluprednisolone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fluprednisolone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fluprednisolone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fluprednisolone supplier is an individual or a company that provides Fluprednisolone active pharmaceutical ingredient (API) or Fluprednisolone finished formulations upon request. The Fluprednisolone suppliers may include Fluprednisolone API manufacturers, exporters, distributors and traders.
click here to find a list of Fluprednisolone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Fluprednisolone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fluprednisolone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fluprednisolone GMP manufacturer or Fluprednisolone GMP API supplier for your needs.
A Fluprednisolone CoA (Certificate of Analysis) is a formal document that attests to Fluprednisolone's compliance with Fluprednisolone specifications and serves as a tool for batch-level quality control.
Fluprednisolone CoA mostly includes findings from lab analyses of a specific batch. For each Fluprednisolone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fluprednisolone may be tested according to a variety of international standards, such as European Pharmacopoeia (Fluprednisolone EP), Fluprednisolone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fluprednisolone USP).
