
Synopsis
Synopsis
0
VMF
0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
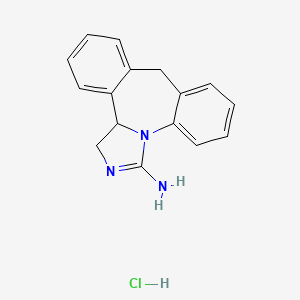

1. 3-amino-9,13b-dihydro-1h-benz(c,f)imidazo(1,5a)azepine
2. Epinastine
3. Flurinol
4. Wal 80
5. Wal 801
6. Wal 801 Cl
7. Wal 801cl
8. Wal-80 Cl
1. Epinastine Hcl
2. 108929-04-0
3. Alesion
4. Elestat
5. Wal-801cl
6. 80012-44-8
7. Wal 801 Cl
8. Epinastine Hydrochloride [jan]
9. De-114
10. Epinastine (hydrochloride)
11. Epinastinehydrochloride
12. Gfm415s5xl
13. Wal-802-cl
14. Wal801
15. Relestat
16. 3-amino-9,13b-dihydro-1h-dibenzo[c,f]imidazo[1,5-a]azepine Hydrochloride
17. 9,13b-dihydro-1h-dibenzo[c,f]imidazo[1,5-a]azepin-3-amine Hydrochloride
18. Dsstox_cid_26502
19. Dsstox_rid_81671
20. Dsstox_gsid_46502
21. Epinastine Hydrochloride (jan)
22. Epinastine Monohydrochloride
23. Chebi:51037
24. Cas-108929-04-0
25. Ncgc00165791-02
26. Unii-gfm415s5xl
27. Flurinol
28. (13brs)-9,13b-dihydro-1h-dibenzo[c,f]imidazo[1,5-a]azepin-3-amine Hydrochloride
29. Alesion (tn)
30. Elestat (tn)
31. Mfcd00933434
32. Epinastin Hydrochloride
33. 9,13b-dihydro-1h-dibenz[cf]imidazo[1,5-a]azepine Hydrochloride
34. Wal-801cl Hcl
35. 3-amino-9,13b-dihydro-1h-dibenz(c,f)imidazo(1,5-a)azepine Monohydrochloride
36. Schembl122749
37. Chembl1200491
38. Dtxsid1046502
39. Epinastine For System Suitability
40. Hy-b0640a
41. Hms3652n21
42. Hms3885b15
43. Bcp12119
44. Ex-a1004
45. Tox21_112263
46. Tox21_500501
47. Epinastine Hydrochloride [mi]
48. S4253
49. Akos015967222
50. Tox21_112263_1
51. Ac-1491
52. Ccg-221805
53. Ks-1159
54. Lp00501
55. Epinastine Hydrochloride [mart.]
56. Ih-dibenz(c,f)imidazo(1,5-a)azepin-3-amine, 9,13b-dihydro-, Monohydrochloride
57. Epinastine Hydrochloride [usp-rs]
58. Epinastine Hydrochloride [who-dd]
59. Ncgc00165791-04
60. Ncgc00261186-01
61. 2,4-diazatetracyclo[12.4.0.02,6.07,12]octadeca-1(18),3,7,9,11,14,16-heptaen-3-amine;hydrochloride
62. Db-040850
63. Cs-0013220
64. E0799
65. Ft-0631049
66. Ft-0700527
67. Sw220223-1
68. Epinastine Hydrochloride [orange Book]
69. D01713
70. Epinastine Hydrochloride [ep Monograph]
71. F17420
72. Epinastine Hydrochloride, >=98% (hplc), Solid
73. A857231
74. J-002209
75. Q27122286
76. Epinastine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
77. 1h-dibenz(c,f)imidazo(1,5-a)azepin-3-amine, 9,13b-dihydro-, Hydrochloride (1:1)
78. 3-amino-9,13b-dihydro-1h-dibenz(c,f)imidazo(1,5-a)azepine Hydrochloride, Dl-
79. Epinastine Hydrochloride, United States Pharmacopeia (usp) Reference Standard
80. 2,4-diazatetracyclo[12.4.0.02,6.07,12]octadeca-1(18),3,7,9,11,14,16-heptaen-3-amine;hydron;chloride
81. Epinastine Hydrochloride For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 285.77 g/mol |
|---|---|
| Molecular Formula | C16H16ClN3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 285.1032752 g/mol |
| Monoisotopic Mass | 285.1032752 g/mol |
| Topological Polar Surface Area | 41.6 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 378 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Elestat |
| PubMed Health | Epinastine (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | ELESTAT (epinastine HCl ophthalmic solution) 0.05% is a clear, colorless, sterile isotonic solution containing epinastine HCl, an antihistamine and an inhibitor of histamine release from the mast cell for topical administration to the eyes. Epinast... |
| Active Ingredient | Epinastine hydrochloride |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 4 | |
|---|---|
| Drug Name | Epinastine hydrochloride |
| Drug Label | Epinastine HCl ophthalmic solution, 0.05% is a clear, colorless, sterile isotonic solution containing epinastine HCl, an antihistamine and an inhibitor of histamine release from the mast cell for topical administration to the eyes.Epinastine HCl is r... |
| Active Ingredient | Epinastine hydrochloride |
| Dosage Form | Solution/drops; Solution |
| Route | ophthalmic; Ophthalmic |
| Strength | 0.05% |
| Market Status | Tentative Approval; Prescription |
| Company | Tesa Pharms; Breckenridge Pharm; Apotex; Sun Pharm Inds; Sandoz |
| 3 of 4 | |
|---|---|
| Drug Name | Elestat |
| PubMed Health | Epinastine (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | ELESTAT (epinastine HCl ophthalmic solution) 0.05% is a clear, colorless, sterile isotonic solution containing epinastine HCl, an antihistamine and an inhibitor of histamine release from the mast cell for topical administration to the eyes. Epinast... |
| Active Ingredient | Epinastine hydrochloride |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 4 | |
|---|---|
| Drug Name | Epinastine hydrochloride |
| Drug Label | Epinastine HCl ophthalmic solution, 0.05% is a clear, colorless, sterile isotonic solution containing epinastine HCl, an antihistamine and an inhibitor of histamine release from the mast cell for topical administration to the eyes.Epinastine HCl is r... |
| Active Ingredient | Epinastine hydrochloride |
| Dosage Form | Solution/drops; Solution |
| Route | ophthalmic; Ophthalmic |
| Strength | 0.05% |
| Market Status | Tentative Approval; Prescription |
| Company | Tesa Pharms; Breckenridge Pharm; Apotex; Sun Pharm Inds; Sandoz |
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21974
Submission : 2008-12-30
Status : Active
Type : II
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.

Date of Issue : 2022-07-07
Valid Till : 2025-07-21
Written Confirmation Number : WC-0150
Address of the Firm :

Date of Issue : 2022-08-25
Valid Till : 2025-07-02
Written Confirmation Number : WC-0156
Address of the Firm :

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15003
Submission : 2000-09-11
Status : Active
Type : II

Certificate Number : R1-CEP 2011-311 - Rev 01
Issue Date : 2021-03-17
Type : Chemical
Substance Number : 2411
Status : Valid

Registration Number : 224MF10100
Registrant's Address : Binger Strasse 173,55216 Ingelheim am Rhein
Initial Date of Registration : 2012-05-23
Latest Date of Registration : --

Registrant Name : Boehringer Ingelheim Korea Ltd.
Registration Date : 2013-01-09
Registration Number : 20130109-194-I-63-01
Manufacturer Name : Boehringer Ingelheim GmbH & Co.KG
Manufacturer Address : Binger Strasse 173 55216 Ingelheim am Rhein, Germany


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22854
Submission : 2009-06-22
Status : Active
Type : II

Date of Issue : 2022-08-08
Valid Till : 2025-07-14
Written Confirmation Number : WC-0168
Address of the Firm :


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21851
Submission : 2008-08-01
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2020-05-27
Pay. Date : 2020-05-19
DMF Number : 21830
Submission : 2008-07-28
Status : Active
Type : II

Registration Number : 226MF10231
Registrant's Address : Via Delle Arti 33, 20863 Concorezzo (MB), Italy
Initial Date of Registration : 2014-12-08
Latest Date of Registration : --

Registrant Name : Insung Trading Co., Ltd.
Registration Date : 2019-05-27
Registration Number : 20130731-194-I-355-03(6)
Manufacturer Name : ICROM SpA
Manufacturer Address : Concorezzo(MB)-Via Delle Arti,33,Italy


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40256
Submission : 2024-08-07
Status : Active
Type : II


Registration Number : 218MF10959
Registrant's Address : 343, Sapyeong-daero, Seocho-gu, Seoul, Korea
Initial Date of Registration : 2006-12-01
Latest Date of Registration : --

Registrant Name : Jeil Pharmaceutical Co., Ltd.
Registration Date : 2013-02-15
Registration Number : 20130215-194-I-243-02
Manufacturer Name : Chongqing Succeway Pharmaceutical Co., Ltd. @Jeil Pharmaceutical Co., Ltd. @[Starting material (epinastin bromide) manufacturer①] Pharmacostec Co., Ltd. @[Starting material (epinastin bromide) manufacturer②] Dong-in Chemical Co., Ltd. @[Starting material
Manufacturer Address : Chongqing Jinlong Industrial District, China@7 Cheonggang-ga-chang-ro, Baekam-myeon, Cheoin-gu, Yongin-si, Gyeonggi-do (B-dong, etc.)@47 Yakjedanji-ro, Hyangnam-eup, Hwaseong-si, Gyeonggi-do@62 Sandan-ro 16beon-gil, Pyeongtaek-si, Gyeonggi-do@204 Sandan-gil, Jeonui-myeon, Sejong Special Self-Governing City


Registration Number : 305MF10001
Registrant's Address : 1062-8 Honjo, Nishikata-cho, Tochigi City, Tochigi Prefecture
Initial Date of Registration : 2023-01-11
Latest Date of Registration : --

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21974
Submission : 2008-12-30
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15003
Submission : 2000-09-11
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2020-05-27
Pay. Date : 2020-05-19
DMF Number : 21830
Submission : 2008-07-28
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22854
Submission : 2009-06-22
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21851
Submission : 2008-08-01
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40256
Submission : 2024-08-07
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registration Number : 224MF10100
Registrant's Address : Binger Strasse 173,55216 Ingelheim am Rhein
Initial Date of Registration : 2012-05-23
Latest Date of Registration : 2017-10-06

Registration Number : 222MF10037
Registrant's Address : 326 Yokamachi, Toyama City, Toyama Prefecture
Initial Date of Registration : 2010-01-28
Latest Date of Registration : 2010-01-28

Epinastine hydrochloride (for manufacturing purposes only)
Registration Number : 226MF10049
Registrant's Address : 2-3-5 Shimookui, Toyama City, Toyama Prefecture
Initial Date of Registration : 2014-02-27
Latest Date of Registration : 2014-02-27

Epinastine hydrochloride (for manufacturing purposes only)
Registration Number : 221MF10218
Registrant's Address : 1062-8 Honjo, Nishikata-cho, Tochigi City, Tochigi Prefecture
Initial Date of Registration : 2009-10-07
Latest Date of Registration : 2023-06-14

Epinastine hydrochloride (for manufacturing purposes only)
Registration Number : 305MF10001
Registrant's Address : 1062-8 Honjo, Nishikata-cho, Tochigi City, Tochigi Prefecture
Initial Date of Registration : 2023-01-11
Latest Date of Registration : 2023-10-04

Registration Number : 227MF10217
Registrant's Address : No. 20 Juxian Road, Gedian Economic & Technology Development Area, Hubei, 436070, P. ...
Initial Date of Registration : 2015-08-31
Latest Date of Registration : 2015-08-31

Registration Number : 226MF10231
Registrant's Address : Via Delle Arti 33, 20863 Concorezzo (MB), Italy
Initial Date of Registration : 2014-12-08
Latest Date of Registration : 2020-06-11

Registration Number : 302MF10108
Registrant's Address : 47, Jeyakdanji-ro, Hyangnam-eup, Hwaseong-si, Gyeonggi-do, Republic of Korea
Initial Date of Registration : 2020-09-08
Latest Date of Registration : 2020-09-08

Registration Number : 302MF10008
Registrant's Address : 47, Jeyakdanji-ro, Hyangnam-eup, Hwaseong-si, Gyeonggi-do, Republic of Korea
Initial Date of Registration : 2020-01-10
Latest Date of Registration : 2020-01-10

Epinastine hydrochloride (C) (for manufacturing purposes only)
Registration Number : 229MF10196
Registrant's Address : 2-3-5 Shimookui, Toyama City, Toyama Prefecture
Initial Date of Registration : 2017-11-07
Latest Date of Registration : 2017-11-07

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?