

Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Bravelle
2. Fertinex
3. Follicle Stimulating Hormone, Human Urine
4. Follicle-stimulating Hormone, Human Urine
5. Follitrin
6. High Purity, Metrodin
7. Human Fsh, Urinary
8. Metrodin
9. Metrodin High Purity
10. Metrodin Hp
11. Neo Fertinorm
12. Urinary Human Fsh
1. 97048-13-0
2. 146479-72-3
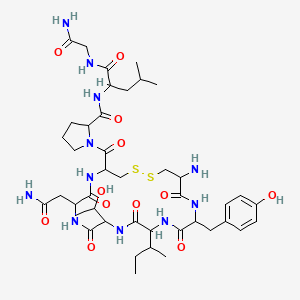
3. 1-[19-amino-7-(2-amino-2-oxoethyl)-13-butan-2-yl-10-(1-hydroxyethyl)-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[1-[(2-amino-2-oxoethyl)amino]-4-methyl-1-oxopentan-2-yl]pyrrolidine-2-carboxamide
4. Fertinex; Fertinorm; Metrodin; Orgafol; Urofollitrophin
5. 26995-91-5
6. Schembl19712185
7. Dtxsid10869286
8. Q4006490
9. 1-{19-amino-7-(2-amino-2-oxoethyl)-13-(butan-2-yl)-10-(1-hydroxyethyl)-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carbonyl}prolylleucylglycinamide
| Molecular Weight | 980.2 g/mol |
|---|---|
| Molecular Formula | C42H65N11O12S2 |
| XLogP3 | -1.5 |
| Hydrogen Bond Donor Count | 12 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 15 |
| Exact Mass | 979.42555890 g/mol |
| Monoisotopic Mass | 979.42555890 g/mol |
| Topological Polar Surface Area | 427 Ų |
| Heavy Atom Count | 67 |
| Formal Charge | 0 |
| Complexity | 1790 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 10 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For treatment of female infertility
FDA Label
Treatment of hypogonadotrophic hypogonadism, Treatment of female infertility
Used for the treatment of female infertility, urofollitropin or follicle stimulating hormone (FSH) stimulates ovarian follicular growth in women who do not have primary ovarian failure. FSH, the active component of urofollitropin is the primary hormone responsible for follicular recruitment and development.
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03G - Gonadotropins and other ovulation stimulants
G03GA - Gonadotropins
G03GA04 - Urofollitropin
Absorption
74%
Route of Elimination
Via liver and kidneys
Volume of Distribution
Time to peak in plasma: IM: 17 hours (single dose), 11 hours (multiple doses) SubQ: 21 hours (single dose), 10 hours (multiple doses)
Circulation half life of 3-4 hours, elimination half life of 35-40 hours
FSH binds to the follicle stimulating hormone receptor which is a G-coupled transmembrane receptor. Binding of the FSH to its receptor seems to induce phosphorylation and activation of the PI3K (Phosphatidylinositol-3-kinase) and Akt signaling pathway, which is known to regulate many other metabolic and related survival/maturation functions in cells.
Global Sales Information

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
