
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Formalin
2. Formol
3. Methanal
4. Oxomethane
1. Formalin
2. Methanal
3. Paraformaldehyde
4. 50-00-0
5. Formol
6. Methylene Oxide
7. Oxomethane
8. Paraform
9. Formic Aldehyde
10. Oxymethylene
11. Methyl Aldehyde
12. Superlysoform
13. Lysoform
14. Fannoform
15. Formalith
16. Formaldehyde Solution
17. Methaldehyde
18. Oxomethylene
19. Formalina
20. Morbicid
21. Karsan
22. 30525-89-4
23. Formaldehyd
24. Formaline
25. Aldehyde Formique
26. Fyde
27. Formaldehyde, Gas
28. Formalin 40
29. Aldeide Formica
30. Oplossingen
31. Dormol
32. Formalin-loesungen
33. Rcra Waste Number U122
34. Aldehyd Mravenci
35. Ch2o
36. Un 2209 (formalin)
37. Formaldehyde (gas)
38. Formaline [german]
39. Nci-c02799
40. Formalina [italian]
41. Oplossingen [dutch]
42. Hcho
43. Caswell No. 465
44. Formyl Group
45. Fordor
46. Un 1198
47. Aldehyd Mravenci [czech]
48. Aldeide Formica [italian]
49. Aldehyde Formique [french]
50. Formalin-loesungen [german]
51. Formaldehyd [czech, Polish]
52. Ccris 315
53. Nsc 298885
54. Formaldehyde [usp]
55. Hsdb 164
56. Aldehyde Formique [iso-french]
57. Bfv
58. Formaldehyde, Solution
59. Chebi:16842
60. Ai3-26806
61. Un1198
62. Un2209
63. Rcra Waste No. U122
64. Epa Pesticide Chemical Code 043001
65. Nsc-298885
66. Dtxsid7020637
67. Formalin Solution
68. Polyformaldehyde
69. Formaldehyde, Para
70. Formaldehyde (usp)
71. 1hg84l3525
72. Formaldehyde, Solution (37% To 50%)
73. Formalde-fresh Solution
74. Dsstox_cid_637
75. Formalin, Buffered, 10%
76. Dsstox_rid_82549
77. Paraformic Aldehyde
78. Dsstox_gsid_47796
79. Formaldehyde, 37% By Weight
80. Formaldehyde, 40% By Volume
81. Formaldehyd (czech, Polish)
82. Formalde-fresh Solution, Buffered
83. Formalaz
84. Formaldehyde, Solutions, Flammable [un1198] [flammable Liquid]
85. Mfcd00003274
86. Mfcd00133991
87. Formaldehyde, Solutions With Not <25% Formaldehyde [un2209] [corrosive]
88. Buffer Solution, Ph 4.00, Color-coded Red
89. Formic Aldehyde
90. Formaidehyde
91. Formaldeyde
92. Formaldhyde
93. Methanon
94. Paraformaldehyd
95. Paraformaldehye
96. Formadehyde
97. Formaldehye
98. Formalinum
99. Durine
100. Paraformaidehyde
101. Para Formaldehyde
102. Paraform-aldehyde
103. Para-formaldehyde
104. Formalin Solution, Neutral Buffered, 10%, Histological Tissue Fixative
105. Unii-1hg84l3525
106. F-gen
107. Hyperband (tn)
108. Formalin [jan]
109. Einecs 200-001-8
110. Hcoh
111. Floguard 1015
112. Formalin (jp17)
113. Carbon-monoxide
114. Hercules 37m6-8
115. H2co
116. Wln: Vhh
117. Formaldehyde [bsi:iso]
118. Formaldehyde [ii]
119. Formaldehyde [mi]
120. Paraformaldehyde (jp17)
121. Short-chain Fatty Aldehyde
122. Bmse000256
123. Epitope Id:116196
124. Ec 200-001-8
125. Formaldehyde [iarc]
126. Formaldehyde [inci]
127. Formaldehyde, 4% In Pbs
128. Formaldehyde, Methanol-free
129. A Short-chain Fatty Aldehyde
130. Chembl1255
131. Formaldehyde [vandf]
132. Formaldehyde Solution, 10%
133. Bidd:er0493
134. Gtpl4196
135. Amy6741
136. Chebi:188447
137. Formaldehyde, As Formalin Solution
138. Formaldehyde, Solutions, Flammable
139. Str00013
140. Tox21_111160
141. Tox21_302438
142. Fm 282
143. Nsc298885
144. Akos008967440
145. Bufferpac™ Color-coded Solutions
146. Db03843
147. Formaldehyde, 37% In Aqueous Solution
148. Na 9202
149. Un 1016
150. Un 2209
151. Formaldehyde Solution 37 Wt. % In H2o
152. Ncgc00255116-01
153. 8013-13-6
154. Bp-21234
155. E240
156. Formaldehyde;formaldehyde [bsi:iso];methanal;formaldehyde (act. 37%);formalin;formaldehyde Formaldehyde [bsi:iso] Methanal Formaldehyde (act. 37%) Formalin
157. Formalin Solution, Neutral Buffered, 10%
158. Formaldehyde (37per Cent W/w Aq. Soln.)
159. Formaldehyde Solution, 37 Wt. % In H2o
160. F0622
161. Ft-0626522
162. Ft-0689115
163. P0018
164. Formaldehyde Solution Acs 37 Wt. % In H2o
165. C00067
166. D00017
167. D01494
168. A827922
169. Formaldehyde Solution, Tested According To Ph.eur.
170. Q161210
171. Sr-01000944454
172. Formaldehyde, Solutions With Not <25% Formaldehyde
173. Sr-01000944454-1
174. Q27110014
175. Formaldehyde Solution, 10% W/w In 84.8 - 94.2% H2o
176. Paraformaldehyde, 16% W/v Aqueous Solution, Methanol Free
177. Formaldehyde Neutral Buffer Solution, 3.7% Formaldehyde In H2o
178. Formaldehyde Neutral Buffer Solution, 7.5% Formaldehyde In H2o
179. Formaldehyde Solution, Puriss. P.a., Acs Reagent, >=36.5%
180. Formaldehyde Solution, For Molecular Biology, 36.5-38% In H2o
181. Formaldehyde Solution, Meets Analytical Specification Of Usp, >=34.5 Wt. %
182. Formaldehyde Solution, Puriss., 37.0%, Contains 6.0-9.0% Methanol
183. Formaldehyde Solution, Ar, Contains 5-8% Methanol As Stabilizer, 37-41 % (w/v)
184. Formaldehyde Solution, Contains 10-15% Methanol As Stabilizer, 37 Wt. % In H2o
185. Formaldehyde Solution, For Molecular Biology, Bioreagent, >=36.0% In H2o (t)
186. Formaldehyde Solution, Jis Special Grade, 36.0-38.0%, Contains Methanol As Stabilizer
187. Formaldehyde Solution, Lr, Contains 5-8% Methanol As Stabilizer, 37-41 % (w/v)
188. Formaldehyde Solution, Saj First Grade, >=35.0%, Contains Methanol As Stabilizer
189. Formalin Solution, Neutral Buffered, 10%, Case Of 24 X 60 Ml, Histological Tissue Fixative
190. Formalin Solution, Neutral Buffered, 10%, Case Of 48 X 15 Ml, Histological Tissue Fixative
191. Formaldehyde Solution, Acs Reagent, 37 Wt. % In H2o, Contains 10-15% Methanol As Stabilizer (to Prevent Polymerization)
192. Formaldehyde Solution, Meets Analytical Specification Of Ph.??eur., Bp, 35 Wt. %, Contains 10% Methanol As Stabilizer
193. Formaldehyde Solution, Meets Usp Testing Specifications, Contains 9.0-15% Methanol As Stabilizer
194. Formaldehyde Solution, Stabilized With Methanol, ~37 Wt. % In H2o, Certified Reference Material
195. Formalin Solution, Neutral Buffered, 10%, Case Of 24 X 120 Ml, Histological Tissue Fixative
| Molecular Weight | 30.026 g/mol |
|---|---|
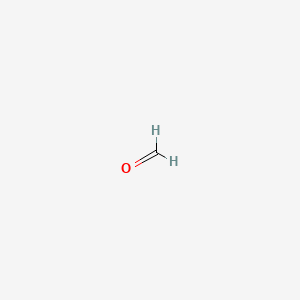
| Molecular Formula | CH2O |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 30.010564683 g/mol |
| Monoisotopic Mass | 30.010564683 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Disinfectants; Fixatives
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/Formaldehyde/ is used for humans as a treatment for athlete's foot, in cough drops, skin disinfectants, mouthwashes, spermatocide creams, as a disinfectant for vasectomies and root canals, and formerly to sterilize certain cysts before surgical removal. /Former use/
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:981
MEDICATION (VET): For various skin diseases of large animals & demodectic mange in dog /soln, USP/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1091
MEDICATION (VET): Antiseptics, fumigant, has been used in tympany, diarrhea, mastitis, pneumonia, internal bleeding.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 751
For more Therapeutic Uses (Complete) data for FORMALDEHYDE (7 total), please visit the HSDB record page.
Anti-Infective Agents, Local
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
In dentistry, it has been used as an obtundent for sensitive dentine & as an antiseptic in mummifying pastes & for root canals. /Former use/
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 1655
Active ingredient of contraceptive creams.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1211
Paraformaldehyde should not be incorporated into any root canal sealer.
BLOCK RM ET AL; ORAL SURG, ORAL MED ORAL PATHOL 50 (4): 350-60 (1980)
Approximate Minimum Lethal Dose (MLD) (150-lb man): 30 mL
Arena, J. M. Poisoning: Toxicology, Symptoms, Treatments. Fourth Edition. Springfield, Illinois: Charles C. Thomas, Publisher, 1979., p. 97
Male single oral ingestion 517 mg/kg /Formalin; from table/
DHHS/ATSDR; Toxicological Profile for Formaldehyde (PB/99/166654) p.116 (1999). Available from, as of December 4, 2014: https://www.atsdr.cdc.gov/toxprofiles/index.asp
Female single oral ingestion 624 mg/kg /Formalin; from table/
DHHS/ATSDR; Toxicological Profile for Formaldehyde (PB/99/166654) p.116 (1999). Available from, as of December 4, 2014: https://www.atsdr.cdc.gov/toxprofiles/index.asp
Lowest lethal dose /for/ human /taking formaldehyde orally was recorded to be/ 36 mg/kg. /From table/
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:967
Use for drying skin before or after surgical removal of warts or where dryness is required.
Disinfectants
Substances used on inanimate objects that destroy harmful microorganisms or inhibit their activity. Disinfectants are classed as complete, destroying SPORES as well as vegetative forms of microorganisms, or incomplete, destroying only vegetative forms of the organisms. They are distinguished from ANTISEPTICS, which are local anti-infective agents used on humans and other animals. (From Hawley's Condensed Chemical Dictionary, 11th ed) (See all compounds classified as Disinfectants.)
Fixatives
Agents employed in the preparation of histologic or pathologic specimens for the purpose of maintaining the existing form and structure of all of the constituent elements. Great numbers of different agents are used; some are also decalcifying and hardening agents. They must quickly kill and coagulate living tissue. (See all compounds classified as Fixatives.)
Formaldehyde is absorbed readily from the respiratory and oral tracts and, to a much lesser degree, from the skin. It is the simplest aldehyde and reacts readily with macromolecules, such as proteins and nucleic acids. Inhalational exposure has been reported to result in almost complete absorption. Dermal absorption due to contact with formaldehyde-containing materials (e.g., textiles, permanent-press clothing, cosmetics, or other materials) is of low order of magnitude. ... Formaldehyde normally is converted and excreted as carbon dioxide in the air, as formic acid in the urine, or as one of many breakdown products from one-carbon pool metabolism. As a result of rapid absorption by both the oral and inhalational routes and its rapid metabolism, little or no formaldehyde is excreted unmetabolized. In rats exposed to (14)C-formaldehyde by inhalation, 40% of the radiolabel was excreted in the air and 20% in the urine and feces, whereas 40% remained in the carcass.
Sullivan, J.B., Krieger G.R. (eds). Clinical Environmental Health and Toxic Exposures. Second edition. Lippincott Williams and Wilkins, Philadelphia, Pennsylvania 1999., p. 1008
In rats and mice administered (14)C-formaldehyde intragastrically, 40% of dose... /was/ expired as carbon dioxide, 10% /was/ excreted in urine and 1% in feces after 12 hr; carcasses contained 20% after 24 hr and 10% after 4 days. When female rats were administered (14)C-formaldehyde ip at dose level of 70 mg/kg, 82% of dose was expired as (14)C dioxide and 13-14% was excreted via kidneys... .
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 340
Formaldehyde is absorbed rapidly and almost completely from the rodent intestinal tract. In rats, about 40% of an oral dose of (14)C-formaldehyde (7 mg/kg) was eliminated as (14)C-carbon dioxide within 12 hours, while 10% was excreted in the urine and 1% in the feces. A substantial portion of the radioactivity remained in the carcass as products of metabolic incorporation.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V62 296 (1995)
Four men and two women were exposed to a 1.9 ppm air concentration of formaldehyde in a large walk-in chamber for 40 minutes. Shortly before and shortly after the exposure, venous blood samples were taken from each person (each person served as his/her own control) and the blood was analyzed for formaldehyde content. Mean venous blood formaldehyde concentrations in humans prior to exposure showed a blood concentration of 2.61 + or - 0.41 ug/g of blood. Individual variability was markedly present. Immediately after a 40-minute exposure, mean blood concentration of formaldehyde was 2.77 + or - 0.28 ug/g of blood. There was no significant difference between pre- and postexposure blood concentrations of formaldehyde at the formaldehyde air concentrations tested in this study. This result suggests that formaldehyde was absorbed only into the tissues of the respiratory tract. The absence of increased formaldehyde concentrations in the blood is likely due to its rapid metabolism in these tissues and/or fast reaction with cellular macromolecules.
DHHS/ATSDR; Toxicological Profile for Formaldehyde (PB/99/166654) p.167 (1999). Available from, as of December 4, 2014: https://www.atsdr.cdc.gov/toxprofiles/index.asp
For more Absorption, Distribution and Excretion (Complete) data for FORMALDEHYDE (13 total), please visit the HSDB record page.
Using rubber-dam isolation and an aseptic and standardized endodontic technique, root canal therapy was performed on the right and left fourth mandibular premolars in four dogs. Each tooth was obturated with N2 paste containing 6.5 percent 14C paraformaldehyde. In each dog at 1 hour, 1, 7, 14, and 28 days, blood was drawn and biopsy specimens were taken of the adjacent lymph nodes, liver, and kidney. At 28 days, the animals were killed, and the experimental and adjacent tissues were processed for light microscopy. Histologic examination demonstrated that particles of the paste placed in the root canal were present in pulp, periapical, and periodontal tissues remote from the original site in the root canal. In addition, 14C-labeled paraformaldehyde which was contained in the N2 paste was found in blood, regional lymph nodes, kidney, and liver. The amount of radioactivity in all the body organs decreased with time.
PMID:6935586 Block RM et al; Oral Surg Oral Med Oral Pathol. 1980 Oct;50(4):350-60.
When female rats were administered (14)C-formaldehyde ip at dose level of 70 mg/kg, 82% of dose was expired as (14)carbon dioxide and 13-14% was excreted via kidneys in form of methionine, serine, and formaldehyde-cysteine adduct.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 340
Rats injected ip with 0.26 mg/kg (14)C-labeled formaldehyde ... excreted approx 22% of this dose in the urine over 5 days. Formic acid and a thiazolidine-4-carboxylic acid derivative were identified in urine as formaldehyde metabolites.
PMID:6697422 Hemminki K; Chem-Biol Interact 48 (2): 243-8 (1984)
Several of the urinary excretion products of formaldehyde in rats have been identified after intraperitoneal administration of (14)C-formaldehyde. After injecting Wistar rats with 0.26 mg/kg body weight, ... formate and a sulfur-containing metabolite (thought to be a derivative of thiazolidine-4-carboxylic acid) and products presumed to result from one-carbon metabolism /were detected/. Thiazolidine-4-carboxylate, which is formed via the nonenzymatic condensation of formaldehyde with cysteine, was not detected in urine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V62 299 (1995)
Formaldehyde absorbed into the bloodstream is metabolized to formic acid, which is excreted in the urine as the sodium salt or oxidized further to carbon dioxide and water. This detoxification process can deal efficiently with low concentrations of formaldehyde, but high concentrations cause acidosis and tissue damage.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:985
For more Metabolism/Metabolites (Complete) data for FORMALDEHYDE (13 total), please visit the HSDB record page.
Urine (for formic acid): 80-90 minutes; [TDR, p. 713]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 713
...In several species... formaldehyde has a half-life of only 1 min; but the half-life for formic acid is species dependent.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 339
Formaldehyde is rapidly metabolized with a half-life in the blood of approx 1.5 min. This half-life is based primarily on primate data although available human data are consistent with this observation of a very short half-life. Data from other species suggest that the half-life of formaldehyde is fairly similar in many species.
Sullivan, J.B., Krieger G.R. (eds). Clinical Environmental Health and Toxic Exposures. Second edition. Lippincott Williams and Wilkins, Philadelphia, Pennsylvania 1999., p. 1008
Formaldehyde is thought to act via sensory nerve fibers that signal through the trigeminal nerve to reflexively induce bronchoconstriction through the vagus nerve.
Klaassen, C.D. (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001., p. 1005
Exposure to formaldehyde, a known air toxic, is associated with cancer and lung disease. Despite the adverse health effects of formaldehyde, the mechanisms underlying formaldehyde-induced disease remain largely unknown. Research has uncovered microRNAs (miRNAs) as key posttranscriptional regulators of gene expression that may influence cellular disease state. Although studies have compared different miRNA expression patterns between diseased and healthy tissue, this is the first study to examine perturbations in global miRNA levels resulting from formaldehyde exposure. We investigated whether cellular miRNA expression profiles are modified by formaldehyde exposure to test the hypothesis that formaldehyde exposure disrupts miRNA expression levels within lung cells, representing a novel epigenetic mechanism through which formaldehyde may induce disease. Human lung epithelial cells were grown at air-liquid interface and exposed to gaseous formaldehyde at 1 ppm for 4 hr. Small RNAs and protein were collected and analyzed for miRNA expression using microarray analysis and for interleukin (IL-8) protein levels by enzyme-linked immunosorbent assay (ELISA). RESULTS: Gaseous formaldehyde exposure altered the miRNA expression profiles in human lung cells. Specifically, 89 miRNAs were significantly down-regulated in formaldehyde-exposed samples versus controls. Functional and molecular network analysis of the predicted miRNA transcript targets revealed that formaldehyde exposure potentially alters signaling pathways associated with cancer, inflammatory response, and endocrine system regulation. IL-8 release increased in cells exposed to formaldehyde, and results were confirmed by real-time polymerase chain reaction. Formaldehyde alters miRNA patterns that regulate gene expression, potentially leading to the initiation of a variety of diseases.
PMID:21147603 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3080931 Rager JE et al; Environ Health Perspect 119 (4): 494-500 (2011)
Formaldehyde at high concentrations is a contributor to air pollution. It is also an endogenous metabolic product in cells, and when beyond physiological concentrations, has pathological effects on neurons. Formaldehyde induces mis-folding and aggregation of neuronal tau protein, hippocampal neuronal apoptosis, cognitive impairment and loss of memory functions, as well as excitation of peripheral nociceptive neurons in cancer pain models. Intracellular calcium ([Ca(2+)](i)) is an important intracellular messenger, and plays a key role in many pathological processes. The present study aimed to investigate the effect of formaldehyde on [Ca(2+)](i) and the possible involvement of N-methyl-D-aspartate receptors (NMDARs) and T-type Ca(2+) channels on the cell membrane. METHODS: Using primary cultured hippocampal neurons as a model, changes of [Ca(2+)](i) in the presence of formaldehyde at a low concentration were detected by confocal laser scanning microscopy. Formaldehyde at 1 mmol/L approximately doubled [Ca(2+)](i). (2R)-amino-5-phosphonopentanoate (AP5, 25 umol/L, an NMDAR antagonist) and mibefradil (MIB, 1 umol/L, a T-type Ca(2+) channel blocker), given 5 min after formaldehyde perfusion, each partly inhibited the formaldehyde-induced increase of [Ca(2+)](i), and this inhibitory effect was reinforced by combined application of AP5 and MIB. When applied 3 min before formaldehyde perfusion, AP5 (even at 50 umol/L) did not inhibit the formaldehyde-induced increase of [Ca(2+)](i), but MIB (1 umol/L) significantly inhibited this increase by 70%. These results suggest that formaldehyde at a low concentration increases [Ca(2+)](i) in cultured hippocampal neurons; NMDARs and T-type Ca(2+) channels may be involved in this process.
PMID:23160928 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5561821 Chi YN et al; Neurosci Bull 28 (6): 715-22 (2012)
/The purpose of this study was/ to study the role of poly (ADP-ribose) polymerase-l (PARP-1) in formaldehyde-induced DNA damage response in human bronchial epithelial (HBE) cells and to investigate the mechanism of formaldehyde carcinogenicity. The protein levels were measured by Western blot. The interaction between different proteins was determined by co-immunoprecipitation assay. The chemical inhibitor was used to confirm the relationship between PARP-1 and DNA damage repair. After being exposed to different concentrations of formaldehyde for 4 hr, HBE cells showed no significant changes in cell viability. Cell viability was significantly reduced after 24-hr exposure to 80 and 160 umol/L formaldehyde (P < 0.05). The 10 umol/L formaldehyde resulted in significant increases in the protein levels of PARP-1 and XRCC-1. However, 80 umol/L formaldehyde led to a significant decrease in the protein level of PARP-1 of 124 KD molecular weight but a significant increase in the protein level of PARP-1 of 89 KD molecular weight; there was no significant change in the protein level of XRCC-1. The co-immunoprecipitation assay showed that 10 umol/L formaldehyde induced increased binding between PARP-1 and XRCC-1, but 80 umol/L formaldehyde led to no significant change in binding between PARP-1 and XRCC-1. Here, we confirmed the role of 10 umol/L formaldehyde in strand breaks by comet assay which showed an increase in the tail DNA content of HBE cells after 4-h formaldehyde exposure. No significant difference was observed in tail DNA content between treated HBE cells and control cells at 2 h after formaldehyde was removed. Moreover, compared with control, inhibition of PARP-1 induced a significant increase in tail DNA content, and a significant difference was observed in tail DNA content between inhibited HBE cells and control cells at 2 h after formaldehyde was removed. Inhibition of PARP-1 significantly reduced DNA repair capacity. PARP-1 mediated the repair of DNA damage induced by low-concentration formaldehyde through recruiting XRCC-1 protein, and may be involved in the regulation of cell apoptosis induced by high-concentration formaldehyde.
PMID:25169219 Jia X et al; Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 32 (6): 401-5 (2014)
Peroxiredoxin 2 (Prx2), a member of the peroxiredoxin family, regulates numerous cellular processes through intracellular oxidative signal transduction pathways. Formaldehyde (FA)-induced toxic damage involves reactive oxygen species (ROS) that trigger subsequent toxic effects and inflammatory responses. The present study aimed to investigate the role of Prx2 in the development of bone marrow toxicity caused by FA and the mechanism underlying FA toxicity. According to the results of the preliminary investigations, the mice were divided into four groups (n=6 per group). One group was exposed to ambient air and the other three groups were exposed to different concentrations of FA (20, 40, 80 mg/cu m) for 15 days in the respective inhalation chambers, for 2 hr a day. At the end of the 15-day experimental period, all of the mice were sacrificed and bone marrow cells were obtained. Cell samples were used for the determination of pathology, glutathione peroxidase (GSH-Px) activity and myeloperoxidase (MPO) activity and protein expression; as well as for the determination of DNA damage and Prx2 expression. The results revealed an evident pathological change in the FA-treated groups, as compared with the controls. In the FA treatment group GSH-Px activity was decreased, while MPO activity and protein expression were increased. The rate of micronucleus and DNA damage in the FA-treated groups was also increased and was significantly different compared with the control, while the expression of Prx2 was decreased. The present study suggested that at certain concentrations, FA had a toxic effect on bone marrow cells and that changes in the Prx2 expression are involved in this process.
PMID:25109304 Yu G et al; Mol Med Rep 10 (4): 1915-20 (2014)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
35
PharmaCompass offers a list of Formaldehyde API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Formaldehyde manufacturer or Formaldehyde supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Formaldehyde manufacturer or Formaldehyde supplier.
PharmaCompass also assists you with knowing the Formaldehyde API Price utilized in the formulation of products. Formaldehyde API Price is not always fixed or binding as the Formaldehyde Price is obtained through a variety of data sources. The Formaldehyde Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Formaldehyde manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Formaldehyde, including repackagers and relabelers. The FDA regulates Formaldehyde manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Formaldehyde API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Formaldehyde manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Formaldehyde supplier is an individual or a company that provides Formaldehyde active pharmaceutical ingredient (API) or Formaldehyde finished formulations upon request. The Formaldehyde suppliers may include Formaldehyde API manufacturers, exporters, distributors and traders.
click here to find a list of Formaldehyde suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Formaldehyde Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Formaldehyde GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Formaldehyde GMP manufacturer or Formaldehyde GMP API supplier for your needs.
A Formaldehyde CoA (Certificate of Analysis) is a formal document that attests to Formaldehyde's compliance with Formaldehyde specifications and serves as a tool for batch-level quality control.
Formaldehyde CoA mostly includes findings from lab analyses of a specific batch. For each Formaldehyde CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Formaldehyde may be tested according to a variety of international standards, such as European Pharmacopoeia (Formaldehyde EP), Formaldehyde JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Formaldehyde USP).
