
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
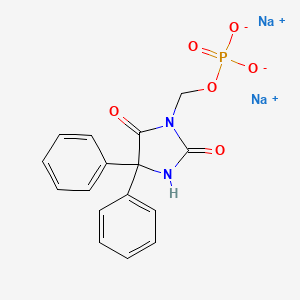

1. 3-(hydroxymethyl)phenytoin Disodium Phosphate
2. 3-(hydroxymethyl)phenytoin Phosphate Ester
3. Acc 9653
4. Acc-9653
5. Cerebyx
6. Fosphenytoin
7. Fosphenytoin, Disodium Salt
8. Hmpdp
9. Prodilantin
1. 92134-98-0
2. Fosphenytoin Disodium Salt
3. Cerebyx
4. Pro-epanutin
5. Ci 982
6. Ci-982
7. Fosphenytoin (sodium)
8. Acc-9653
9. Acc-9653-010 (sodium Salt)
10. Disodium;(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methyl Phosphate
11. Fosphenytoin (disodium)
12. 3-(hydroxymethyl)-5,5-diphenylhydantoin, Disodium Phosphate (ester)
13. 7vlr55452z
14. 92134-98-0 (sodium)
15. Acc-9653-010
16. 2,4-imidazolidinedione, 5,5-diphenyl-3-((phosphonooxy)methyl)-, Disodium Salt
17. Fosphenytoin Disodium
18. Sodium (2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methyl Phosphate
19. Fosphenytoinsodium
20. Acc 9653
21. Fosphenytoin Sodium [usan]
22. Fosphenytoin_sodium
23. Fosphenytoin Disodium Salt Hydrate
24. Acc 9653-010
25. Fostoin
26. Unii-7vlr55452z
27. Fosphenytoin Sodium [usan:usp]
28. Phosphenytoin Sodium
29. Cerebyx (tn)
30. Fosphenytoin Sodium Salt
31. Chembl919
32. Fosphenytoin Sodium (usp)
33. Schembl119417
34. Npc-06
35. Dtxsid1044271
36. Fosphenytoin Sodium [vandf]
37. Bcp13859
38. Fosphenytoin Sodium [mart.]
39. Disodium,(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methyl Phosphate
40. Fosphenytoin Sodium [usp-rs]
41. Fosphenytoin Sodium [who-dd]
42. 5,5-diphenyl-3-[(phosphonooxy)methyl]-2,4-imidazolidinedione Disodium Salt
43. Akos015915510
44. Ac-1705
45. Fosphenytoin Disodium Salt [mi]
46. Fosphenytoin Sodium [orange Book]
47. Fosphenytoin Sodium [usp Impurity]
48. Fosphenytoin Sodium [usp Monograph]
49. Db-057291
50. Ft-0630980
51. D02096
52. D81920
53. A844152
54. Acc 9653 Pound>>acc9653 Pound>>acc-9653
55. Q27268914
56. Disodium (2,5-dioxo-4,4-diphenyl-1-imidazolidinyl)methyl Phosphate
57. Disodium [2,5-bis(oxidanylidene)-4,4-diphenyl-imidazolidin-1-yl]methyl Phosphate
| Molecular Weight | 406.24 g/mol |
|---|---|
| Molecular Formula | C16H13N2Na2O6P |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 406.03066170 g/mol |
| Monoisotopic Mass | 406.03066170 g/mol |
| Topological Polar Surface Area | 122 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 536 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 4 | |
|---|---|
| Drug Name | Cerebyx |
| PubMed Health | fosphenytoin |
| Drug Classes | Anticonvulsant |
| Drug Label | CEREBYX (fosphenytoin sodium injection) is a prodrug intended for parenteral administration; its active metabolite is phenytoin. 1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium, and is referred to as 1 mg phenytoin sodium equiv... |
| Active Ingredient | Fosphenytoin sodium |
| Dosage Form | Injectable |
| 2 of 4 | |
|---|---|
| Drug Name | Fosphenytoin sodium |
| PubMed Health | fosphenytoin |
| Drug Label | Fosphenytoin Sodium Injection, USP is a prodrug intended for parenteral administration; its active metabolite is phenytoin.1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium, and is referred to as 1 mg phenytoin sodium equivalents... |
| Active Ingredient | Fosphenytoin sodium |
| Dosage Form | Injectable |
| 3 of 4 | |
|---|---|
| Drug Name | Cerebyx |
| PubMed Health | fosphenytoin |
| Drug Classes | Anticonvulsant |
| Drug Label | CEREBYX (fosphenytoin sodium injection) is a prodrug intended for parenteral administration; its active metabolite is phenytoin. 1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium, and is referred to as 1 mg phenytoin sodium equiv... |
| Active Ingredient | Fosphenytoin sodium |
| Dosage Form | Injectable |
| 4 of 4 | |
|---|---|
| Drug Name | Fosphenytoin sodium |
| PubMed Health | fosphenytoin |
| Drug Label | Fosphenytoin Sodium Injection, USP is a prodrug intended for parenteral administration; its active metabolite is phenytoin.1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium, and is referred to as 1 mg phenytoin sodium equivalents... |
| Active Ingredient | Fosphenytoin sodium |
| Dosage Form | Injectable |
Sodium Channel Blockers
A class of drugs that act by inhibition of sodium influx through cell membranes. Blockade of sodium channels slows the rate and amplitude of initial rapid depolarization, reduces cell excitability, and reduces conduction velocity. (See all compounds classified as Sodium Channel Blockers.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?