
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
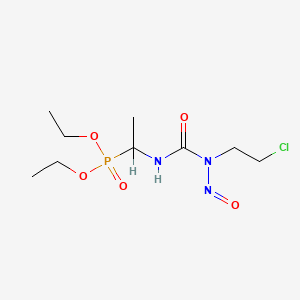

1. Diethyl-1-(3-(2-chloroethyl)-3-nitrosoureido)ethylphosphonate
2. Muphoran
3. Mustoforan
4. S 10036
5. S-10036
1. 92118-27-9
2. Diethyl (1-(3-(2-chloroethyl)-3-nitrosoureido)ethyl)phosphonate
3. Muphoran
4. Mustophorane
5. Fotemustina
6. Servier-10036
7. S 10036
8. Mustoforan
9. S-10036
10. Fotemustine, (+)-
11. Fotemustine, (-)-
12. Upb2nn83ar
13. Gq7jl9p5i2
14. Qy93p3gn94
15. S10036
16. Phosphonic Acid, (1-((((2-chloroethyl)nitrosoamino)carbonyl)amino)ethyl)-, Diethyl Ester
17. Fotemustinum [latin]
18. Diethyl-1-(3-(2-chloroethyl)-3-nitrosoureido)ethylphosphonate
19. Fotemustina [spanish]
20. Fotemustinum
21. (+-)-diethyl (1-(3-(2-chloroethyl)-3-nitrosoureido)ethyl)phosphonate
22. Diethyl (1-{[(2-chloroethyl)(nitroso)carbamoyl]amino}ethyl)phosphonate
23. 191219-77-9
24. 191220-84-5
25. Phosphonic Acid, (1-((((2-chloroethyl)nitrosoamino)carbonyl)amino)ethyl)-, Diethyl Ester, (+)-
26. Phosphonic Acid, (1-((((2-chloroethyl)nitrosoamino)carbonyl)amino)ethyl)-, Diethyl Ester, (-)-
27. Smr002529685
28. Ccris 6337
29. Fotemustine [inn:ban]
30. Unii-gq7jl9p5i2
31. Fotemustene
32. 1-(2-chloroethyl)-3-(1-diethoxyphosphorylethyl)-1-nitrosourea
33. Hsdb 7762
34. Muphoran (tn)
35. Mfcd00866278
36. Fotemustine (inn/ban)
37. Fotemustine [mi]
38. Unii-upb2nn83ar
39. Fotemustine [inn]
40. Fotemustine [hsdb]
41. Schembl8880
42. Fotemustine [mart.]
43. Unii-qy93p3gn94
44. Fotemustine [who-dd]
45. Mls006010211
46. Mls006010716
47. Chembl549386
48. 6-amino-3-hydroxy(1h)indazole
49. Fotemustine, >=98% (hplc)
50. Dtxsid80869091
51. Chebi:131852
52. Bcp07342
53. Hy-b0733
54. Akos015920275
55. Ds-1395
56. Ncgc00346829-01
57. Ncgc00346829-03
58. Db-057290
59. Ft-0630979
60. D07255
61. 118f279
62. A844148
63. Sr-01000944936
64. Q1439555
65. Sr-01000944936-1
66. Diethyl 1-(3-(2-chloroethyl)-3-nitrosoureido)ethylphosphonate
67. 1-(2-chloroethyl)-3-(1-diethoxyphosphorylethyl)-1-nitroso-urea
68. (+/-)-diethyl (1-(3-(2-chloroethyl)-3-nitrosoureido)ethyl)phosphonate
69. Diethyl (1-{[n-(2-chloroethyl)-n'-oxohydrazinecarbonyl]amino}ethyl)phosphonate
70. Phosphonic Acid, P-(1-((((2-chloroethyl)nitrosoamino)carbonyl)amino)ethyl)-, Diethyl Ester
| Molecular Weight | 315.69 g/mol |
|---|---|
| Molecular Formula | C9H19ClN3O5P |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 315.0750854 g/mol |
| Monoisotopic Mass | 315.0750854 g/mol |
| Topological Polar Surface Area | 97.3 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 334 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic[The Merck Index, Fourteenth Edition (2006)
CD-ROM]
The indication "disseminated malignant melanoma", including cerebral metastases, is currently the preferential indication for fotemustine, administered alone or in combination with other anticancer agents.
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
/EXPERIMENTAL THERAPY:/ ... Patients with progressive glioblastoma multiforme after radiotherapy plus concomitant and/or adjuvant temozolomide received three-weekly doses (100-75 mg/ sq m) of fotemustine followed, after a 5-week rest, by fotemustine (100 mg/sq m) every 3 weeks for < or =1 year. Forty-three patients (29 M, 14 F; median age 51 years, range 34-68; median KPS 90) were enrolled. Progression-free survival at 6 months (PFS-6) was 20.9% (95% CI: 9-33%); three patients (7.1%) had partial response (PR); 15 (34.9%), disease stabilization (SD). The median survival was 6 months (95% CI: 5-7). O6-Methylguanine-DNA methyltransferase (MGMT) promoter status was methylated in 8 (18.6%) and unmethylated in 26 (60.5%) and not assessable in 9 (20.9%) patients, respectively. Disease control was 75% versus 34.6% in methylated and unmethylated MGMT patients (P = 0.044); no significant difference was found between groups for PFS-6 and survival. Grade 3 and 4 thrombocytopenia and neutropenia were observed in 20.9 and 16.3% of patients, during the induction phase, and in 0 and 9.5% patients during the maintenance phase, respectively. The findings of the present trial, that evaluate fotemustine in a homogeneous population, may represent a new benchmark for nitrosourea activity. Moreover, this is the first study to evaluate correlation between MGMT promoter status and outcome of fotemustine for relapsing glioblastoma multiforme previously treated with radiotherapy and temozolomide.
PMID:19169684 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2717374 Brandes AA et al; Cancer Chemother Pharmacol 64 (4): 769-75 (2009).
/EXPERIMENTAL THERAPY:/ ... This study investigated the impact of hepatic arterial chemotherapy in patients with ocular and cutaneous melanoma. In a retrospectively analyzed observational study, 36 consecutive patients with hepatic metastases from ocular or cutaneous melanoma were assigned for surgical hepatic port-catheter implantation. Fotemustine was delivered weekly for a 4-week period, followed by a 5-week rest and a maintenance period every 3 weeks until progression. Overall survival, response and toxicity were analysed and compared. After port-catheter implantation 30/36 patients were finally treated (18 with ocular and 12 with cutaneous melanoma). A median of 8 infusions per patient were delivered (range 3-24). 30% thrombocytopenia grade >or=3, 7% neutropenia grade >or= 3 but no nausea or vomiting grade >or= 3 were encountered. Nine out of 30 patients achieved partial remission, 10/30 stable disease; 11/30 patients were progressive. Median survival for all treated patients was 14 months. Partial remission and stable disease were associated with a survival advantage compared to progressive disease (19 vs. 5 months). No significant difference in survival was observed for ocular versus cutaneous melanoma. Serum LDH was a significant predictor of both response and survival. Hepatic arterial fotemustine chemotherapy was well tolerated. Meaningful response and survival rates were achieved in ocular as well as cutaneous melanoma. Careful patient selection in consideration of extra-hepatic involvement is crucial for the effectiveness of this treatment. Independent from the primary melanoma, it is debatable if patients with highly elevated serum-LDH may benefit from this approach.
PMID:17196362 Siegel R et al; Eur J Surg Oncol 33 (5): 627-32 (2007).
For more Therapeutic Uses (Complete) data for Fotemustine (6 total), please visit the HSDB record page.
Because of the known mutagenic and carcinogenic potential of nitrosoureas, administration to pregnant women is contraindicated. Contraindicated in lactation.
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
As no studies have been conducted, it is not recommended to use fotemustine in children in the present state of knowledge.
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
It is recommended not to administer the product to patients who have already received chemotherapy in the previous 4 weeks (or 6 weeks in the case of previous treatment with a nitrosourea).
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
Treatment can only be considered when the platelet count and/or granulocyte count is acceptable, with minimum values of 100,000/cu mm and 2000/cu mm respectively.
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
For more Drug Warnings (Complete) data for Fotemustine (10 total), please visit the HSDB record page.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AD - Nitrosoureas
L01AD05 - Fotemustine
The pharmacokinetics and metabolism of intravenously infused (14)C-fotemustine (about 100 mg/sq m) were examined in 2 cancer patients. Plasma levels of radioactivity increased to a maximum of 4.1 and 5.5 ug equivalents per g when the infusion stopped then declined triexponentially with mean half-lives of about 1/2, 10 and 80 hr for the initial, mid and terminal phases, respectively. Plasma levels of intact drug were lower, with maximum levels of 1.1 and 2.8 micrograms/ml, and declined monophasically with a half-life of about 24 min. Plasma clearance was high (1426 and 764 mL/min) with the volume of distribution based on areas of 47.7 and 26.4 L. Most of the radioactivity was eliminated in urine (50.1 and 61.3%) over 7 days with smaller amounts in the feces (6.8 and 0.3%) and only minimal quantities (under 0.1%) as expired carbon dioxide. ...
PMID:2145908 Ings RM et al; Eur J Cancer 26 (7): 838-42 (1990).
After administration in /humans/ of the drug labelled with (14)C on the chloroethyl group, the radioactivity is slowly eliminated with a terminal half-life of 83 hours. About 50 to 60% of the radioactivity administered is detected in the urine, 30 to 40% of which is detected during the first 24 hours, but the unchanged molecule is not detected in the urine. 5% of the radioactivity is eliminated in the feces and less than 0.2% in the form of expired CO2.
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
In /humans/, during administration by intravenous infusion, the plasma levels of fotemustine are close to the steady-state value after 45 minutes. After the end of the infusion, plasma levels go down rapidly and three hours later the molecule can no longer be detected in the blood.
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
In animals, the tissue distribution is rapid and very extensive. Fotemustine crosses the blood-brain barrier (2 to 5 minutes after bolus administration in the rat, it is detected in the brain at sufficiently high levels to be active).
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
The pharmacokinetics and metabolism of intravenously infused (14)C-fotemustine (about 100 mg/sq m) were examined in 2 cancer patients. ... Metabolites of fotemustine were identified as chloroethanol and N-nitroso-1-imidazolone-ethyl-diethylphosphonate in plasma and as 1-hydantoin-ethyl-diethyl-phosphonate and acetic acid in urine.
PMID:2145908 Ings RM et al; Eur J Cancer 26 (7): 838-42 (1990).
After administration in /humans/ of the drug labelled with (14)C on the chloroethyl group, the radioactivity is slowly eliminated with a terminal half-life of 83 hours.
New Zealand Ministry of Health, MEDSAFE, New Zealand Medicines and Medical Devices Safety Authority, Information for Health Professionals, Data Sheet for Muphoran (fotemustine, 209 mg powder for injection). Last updated May 2002. Available from, as of November 17, 2009: https://www.medsafe.govt.nz/Profs/Datasheet/m/Muphoraninj.htm
The pharmacokinetics and metabolism of intravenously infused (14)C-fotemustine (about 100 mg/sq m) were examined in 2 cancer patients. Plasma levels of radioactivity increased to a maximum of 4.1 and 5.5 ug equivalents per g when the infusion stopped then declined triexponentially with mean half-lives of about 1/2, 10 and 80 hr for the initial, mid and terminal phases, respectively. ...
PMID:2145908 Ings RM et al; Eur J Cancer 26 (7): 838-42 (1990).
... The mode of cell death in 11 melanoma cell lines upon exposure to temozolomide and fotemustine /is determined/. ... For both temozolomide and fotemustine apoptosis is the dominant mode of cell death. The contribution of necrosis to total cell death varied between 10 and 40%. The O(6)-methylguanine-DNA methyltransferase (MGMT) activity in the cell lines was between 0 and 1100 fmol mg(-1) protein, and there was a correlation between MGMT activity and the level of resistance to temozolomide and fotemustine. MGMT inactivation by O(6)-benzylguanine sensitized all melanoma cell lines expressing MGMT to temozolomideand fotemustine-induced apoptosis, and MGMT transfection attenuated the apoptotic response. This supports that O(6)-alkylguanines are critical lesions involved in the initiation of programmed melanoma cell death. One of the cell lines (MZ7), derived from a patient subjected to DTIC therapy, exhibited a high level of resistance to temozolomide without expressing MGMT. This was related to an impaired expression of MSH2 and MSH6. The cells were not cross-resistant to fotemustine. Although these data indicate that methylating drug resistance of melanoma cells can be acquired by down-regulation of mismatch repair, a correlation between MSH2 and MSH6 expression in the different lines and temozolomide sensitivity was not found. Apoptosis in melanoma cells induced by temozolomide and fotemustine was accompanied by double-strand break formation (as determined by H2AX phosphorylation) and caspase-3 and -7 activation as well as PARP cleavage. For temozolomide, double-strand breaks correlated significantly with the apoptotic response, whereas for fotemustine a correlation was not found. Melanoma lines expressing p53 wild-type were more resistant to temozolomide and fotemustine than p53 mutant melanoma lines, which is in marked contrast to previous data reported for glioma cells treated with temozolomide. Overall, the findings are in line with the model that in melanoma cells temozolomide-induced O(6)-methylguanine triggers the apoptotic (and necrotic) pathway through double-strand breaks, whereas for chloroethylating agents apoptosis is triggered in a more complex manner.
PMID:19127257 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2634706 Naumann SC et al; Br J Cancer. 2009 Jan 27;100(2):322-33 (2009).
Global Sales Information

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?