
Synopsis
Synopsis
0
KDMF
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
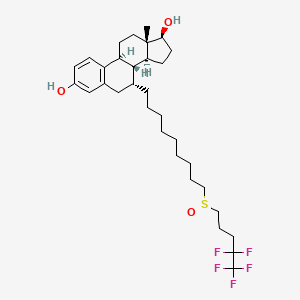

1. 7-(9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl)estra-1,3,5(10)-triene-3,17-diol
2. Faslodex
3. Ici 182,780
4. Ici 182780
5. Ici-182780
6. Ici182780
7. Zm 182780
8. Zm-182780
9. Zm182780
1. 129453-61-8
2. Faslodex
3. Ici 182,780
4. Ici 182780
5. Ici-182780
6. Zm 182780
7. Zd-9238
8. Zm-182780
9. Zd9238
10. Fulvestrant (faslodex)
11. (7r,8r,9s,13s,14s,17s)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol
12. Zd 9238
13. Chembl1358
14. (7r,8r,9s,13s,14s,17s)-13-methyl-7-(9-((4,4,5,5,5-pentafluoropentyl)sulfinyl)nonyl)-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
15. Chebi:31638
16. 22x328qoc4
17. Nsc-759879
18. Estra-1,3,5(10)-triene-3,17-diol, 7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]-, (7alpha,17beta)-
19. Fulvestrant [usan]
20. Dsstox_cid_2369
21. Dsstox_rid_76561
22. Dsstox_gsid_22369
23. (7r,13s,17s)-13-methyl-7-(9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl)-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
24. 7alpha-(9-((4,4,5,5,5-pentafluoropentyl)sulfinyl)nonyl)estra-1,3,5(10)-triene-3,17beta-diol
25. Zd 182780
26. Faslodex (tn)
27. (7alpha,17beta)-7-{9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl}estra-1(10),2,4-triene-3,17-diol
28. (7alpha,17beta)-7-{9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl}estra-1,3,5(10)-triene-3,17-diol
29. 7alpha-[9[(4,4,5,5,5-pentafluropentyl)sulfinyl]nonyl]-estra-1,3,5(10)-triene-3, 17 Beta Diol
30. Estra-1,3,5(10)-triene-3,17-diol, 7-(9-((4,4,5,5,5-pentafluoropentyl)sulfinyl)nonyl)-, (7alpha,17beta)-
31. Cas-129453-61-8
32. Ici182780
33. Fulvestrantum
34. Fluvestrant
35. Unii-22x328qoc4
36. Ccris 8741
37. Faslodex(ici 182,780)
38. Hsdb 7658
39. Ncgc00024964-02
40. Fulvestrant [usan:usp:inn:ban]
41. Mfcd00903953
42. Zd-182780
43. Fulvestrant [mi]
44. Fulvestrant [inn]
45. Fulvestrant [jan]
46. Fulvestrant [vandf]
47. Schembl8209
48. Fulvestrant [mart.]
49. Bidd:pxr0136
50. Fulvestrant [usp-rs]
51. Fulvestrant [who-dd]
52. Mls006010187
53. Bidd:er0348
54. Fulvestrant (jan/usp/inn)
55. Fulvestrant [ema Epar]
56. Fulvestrant, >98% (hplc)
57. Gtpl1015
58. Dtxsid4022369
59. Ex-a959
60. Fulvestrant [orange Book]
61. Ici 182780- Bio-x
62. Fulvestrant [ep Monograph]
63. Fulvestrant [usp Impurity]
64. Hms2090n22
65. Hms3260g10
66. Hms3712a06
67. Fulvestrant [usp Monograph]
68. Tox21_110939
69. Tox21_202604
70. Tox21_303656
71. Tox21_500114
72. Bdbm50169743
73. Nsc719276
74. Akos015895669
75. Tox21_110939_1
76. Ac-4693
77. Bcp9000707
78. Ccg-220082
79. Ccg-221418
80. Cs-1267
81. Db00947
82. Lp00114
83. Nsc 759879
84. Nsc-719276
85. Sdccgsbi-0633685.p001
86. Ici 182 780
87. Ici 182,789
88. Ncgc00164789-02
89. Ncgc00164789-04
90. Ncgc00257357-01
91. Ncgc00260152-01
92. Ncgc00260799-01
93. Ncgc00386134-10
94. (7r,8s,9s,13s,14s,17s)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl) Nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol
95. As-13024
96. Bi167222
97. Hy-13636
98. Smr001456109
99. Bcp0726000227
100. F1144
101. S1191
102. Fulvestrant, Vetec(tm) Reagent Grade, >98%
103. D01161
104. Ab01273957-01
105. Ab01273957-02
106. Ab01273957-03
107. Ab01273957_04
108. 453f618
109. J-005680
110. Q5508491
111. Brd-a85667082-001-12-7
112. Fulvestrant, European Pharmacopoeia (ep) Reference Standard
113. Fulvestrant, United States Pharmacopeia (usp) Reference Standard
114. Fulvestrant For System Suitability, European Pharmacopoeia (ep) Reference Standard
115. (13s,17s)-13-methyl-7-[9-(4,4,5,5,5-pentafluoro-pentane-1-sulfinyl)-nonyl]-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
116. (7?,17?)-7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol
117. (7r,13s,17s)-13-methyl-7-[9-(4,4,5,5,5-pentafluoro-pentane-1-sulfinyl)-nonyl]-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
118. (7r,8r,9s,13s,14s,17s)-13-methyl-7-(9-(4,4,5,5,5-pentafluoropentyldihydrosulfinyl)nonyl)-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
119. (7r,8r,9s,13s,14s,17s)-13-methyl-7-(9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl)-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
120. (7r,8r,9s,13s,14s,17s)-13-methyl-7-[9-(4,4,5,5,5-pentafluoro-pentane-1-sulfinyl)-nonyl]-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
121. (7r,9s,13s,14s,17s)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol
122. 13-methyl-7-[9-(4,4,5,5,5-pentafluoro-pentane-1-sulfinyl)-nonyl]-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
123. 13-methyl-7-[9-(4,5,5,5-pentafluoropentylsulfinyl)nonyl]-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthrene-3,17-diol
124. 7.alpha.-(9-(4,4,5,5,5-pentafluoropentylsulphinyl)nonyl)estra-1,3,5(10)-triene-3,17.beta.-diol
125. 7alpha-[9-(4,4,5,5,5-pentafluoro-pentylsulfinyl)nonyl]estra-1,3,5(10)-triene-3,17beta-diol
126. 7alpha-[9-[(rs)-(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17beta-diol
127. 7alpha-{9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl}estra-1(10),2,4-triene-3,17beta-diol
128. Estra-1,3,5(10)-triene-3,17-diol, 7-(9-((4,4,5,5,5-pentafluoropentyl)sulfinyl)nonyl)-, (7.alpha.,17.beta.)-
129. Estra-1,5(10)-triene-3,17-diol, 7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]-, (7.alpha.,17.beta.)-
| Molecular Weight | 606.8 g/mol |
|---|---|
| Molecular Formula | C32H47F5O3S |
| XLogP3 | 9.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 14 |
| Exact Mass | 606.31660734 g/mol |
| Monoisotopic Mass | 606.31660734 g/mol |
| Topological Polar Surface Area | 76.7 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 854 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Faslodex |
| PubMed Health | Fulvestrant (Injection) |
| Drug Classes | Antiestrogen |
| Drug Label | FASLODEX (fulvestrant) Injection for intramuscular administration is an estrogen receptor antagonist. The chemical name is 7-alpha-[9-(4,4,5,5,5-penta fluoropentylsulphinyl) nonyl]estra-1,3,5-(10)- triene-3,17-beta-diol. The molecular formula is C3... |
| Active Ingredient | Fulvestrant |
| Dosage Form | Injectable |
| Route | Intramuscular |
| Strength | 50mg/ml |
| Market Status | Prescription |
| Company | Astrazeneca |
| 2 of 2 | |
|---|---|
| Drug Name | Faslodex |
| PubMed Health | Fulvestrant (Injection) |
| Drug Classes | Antiestrogen |
| Drug Label | FASLODEX (fulvestrant) Injection for intramuscular administration is an estrogen receptor antagonist. The chemical name is 7-alpha-[9-(4,4,5,5,5-penta fluoropentylsulphinyl) nonyl]estra-1,3,5-(10)- triene-3,17-beta-diol. The molecular formula is C3... |
| Active Ingredient | Fulvestrant |
| Dosage Form | Injectable |
| Route | Intramuscular |
| Strength | 50mg/ml |
| Market Status | Prescription |
| Company | Astrazeneca |
Antineoplastic Agents; Hormonal Estrogen Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Fulvestrant is indicated for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following antiestrogen therapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Faslodex (Fulvestrant) (June 2006). Available from, as of November 13, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1615
/Fulvestrant is contraindicated in/ pregnancy, known hypersensitivity to fulvestrant, benzyl alcohol, or any ingredient in the formulation.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1073
Because fulvestrant is administered by IM injection, the drug should not be used in patients with bleeding diatheses or thrombocytopenia or in those receiving anticoagulant therapy.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
The most common adverse effects of fulvestrant are adverse GI effects (e.g., nausea, vomiting, constipation, diarrhea, abdominal pain), headache, back pain, vasodilation (hot flushes), and pharyngitis, which occurred in approximately 52, 15, 14, 18, and 16% of patients, respectively, who received the drug in clinical studies.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
Other adverse effects occurring in 5-23% of patients receiving fulvestrant (in order of descending frequency) include asthenia, pain, nutritional disorders, bone pain, dyspnea, injection site pain, increased cough, pelvic pain, anorexia, peripheral edema, rash, chest pain, flu syndrome, dizziness, insomnia, fever, paresthesia, urinary tract infection, depression, anxiety, and sweating. Injection site reactions with mild transient pain and inflammation were reported in 7% of patients receiving a single 5-mL injection of fulvestrant in one study and in 27% of those who received two 2.5-mL injections of the drug in another study.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
For more Drug Warnings (Complete) data for FULVESTRANT (7 total), please visit the HSDB record page.
For the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy, as monotherapy or in combination with other antineoplastic agents.
FDA Label
Faslodex is indicated
- as monotherapy for the treatment of estrogen receptor positive, locally advanced or metastatic breast cancer in postmenopausal women:
- not previously treated with endocrine therapy, or
- with disease relapse on or after adjuvant antiestrogen therapy, or disease progression on antiestrogen therapy.
- in combination with palbociclib for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in women who have received prior endocrine therapy.
In pre- or perimenopausal women, the combination treatment with palbociclib should be combined with a luteinizing hormone releasing hormone (LHRH) agonist.
Fulvestrant for intramuscular administration is an estrogen receptor antagonist without known agonist effects.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Estrogen Receptor Antagonists
Compounds and drugs that bind to and block or inhibit the activation of ESTROGEN RECEPTORS. (See all compounds classified as Estrogen Receptor Antagonists.)
L02BA03
L02BA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BA - Anti-estrogens
L02BA03 - Fulvestrant
Route of Elimination
Fulvestrant was rapidly cleared by the hepatobiliary route with excretion primarily via the feces (approximately 90%). Renal elimination was negligible (less than 1%).
Volume of Distribution
3 to 5 L/kg
Peak plasma concentrations of fulvestrant are attained approximately 7 days after IM administration and persist for at least 1 month. Steady-state plasma fulvestrant concentrations usually are achieved within 3-6 months when the drug is administered once-monthly by IM injection.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
Fulvestrant appears to be rapidly and extensively distributed, principally into the extravascular space
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
99% (mainly VLDL, LDL, and HDL lipoprotein fractions).
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
Has been shown to cross the placenta and distribute into milk in rats.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
For more Absorption, Distribution and Excretion (Complete) data for FULVESTRANT (8 total), please visit the HSDB record page.
Metabolism of fulvestrant appears to involve combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids, including oxidation, aromatic hydroxylation, conjugation with glucuronic acid and/or sulphate at the 2, 3 and 17 positions of the steroid nucleus, and oxidation of the side chain sulphoxide. Identified metabolites are either less active or exhibit similar activity to fulvestrant in antiestrogen models. Studies using human liver preparations and recombinant human enzymes indicate that cytochrome P-450 3A4 (CYP 3A4) is the only P-450 isoenzyme involved in the oxidation of fulvestrant; however, the relative contribution of P-450 and non-P-450 routes in vivo is unknown.
Biotransformation and disposition of fulvestrant in humans have been determined following intramuscular and intravenous administration of 14C-labeled fulvestrant. Metabolism of fulvestrant appears to involve combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids, including oxidation, aromatic hydroxylation, conjugation with glucuronic acid and/or sulphate at the 2, 3 and 17 positions of the steroid nucleus, and oxidation of the side chain sulphoxide.
US Natl Inst Health; DailyMed. Current Medication Information for Faslodex (Fulvestrant) (June 2006). Available from, as of November 13, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1615
Metabolites of fulvestrant exhibit pharmacologic activity that is similar to or less than that of the parent compound.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
In vitro studies indicate that CYP3A4 is the only enzyme involved in fulvestrant oxidation; however, the relative contribution of CYP and non-CYP routes in vivo currently is not known.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
40 days
The elimination half-life of fulvestrant is about 40 days.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
Fulvestrant competitively and reversibly binds to estrogen receptors present in cancer cells and achieves its anti-estrogen effects through two separate mechanisms. First, fulvestrant binds to the receptors and downregulates them so that estrogen is no longer able to bind to these receptors. Second, fulvestrant degrades the estrogen receptors to which it is bound. Both of these mechanisms inhibit the growth of tamoxifen-resistant as well as estrogen-sensitive human breast cancer cell lines.
Fulvestrant, a 7(alpha)-alkylsulfinyl analog of estradiol, is an estrogen antagonist. Data from animal studies indicate that fulvestrant does not possess estrogen-agonist activity. The drug competitively binds to and downregulates estrogen receptors in human breast cancer cells. Fulvestrant has been shown to inhibit the growth of tamoxifen-resistant as well as estrogen-sensitive human breast cancer (MCF-7) cell lines in vitro and in vivo. Data from studies in animals indicate that the drug also may block the uterotropic action of estradiol. Fulvestrant does not appear to exhibit peripheral steroidal effects in postmenopausal women, as evidenced by an absence of appreciable changes in plasma concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) after receiving 250 mg of fulvestrant IM monthly.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1074
The efferent ductules express the highest amount of estrogen receptors ESR1 (ERalpha) and ESR2 (ERbeta) within the male reproductive tract. Treatment of rats with the antiestrogen fulvestrant (ICI 182,780) causes inhibition of fluid reabsorption in the efferent ductules, leading to seminiferous tubule atrophy and infertility. To provide a more comprehensive knowledge about the molecular targets for estrogen in the rat efferent ductules, /the authors/ investigated the effects of ICI 182,780 treatment on gene expression using a microarray approach. Treatment with ICI 182,780 increased or reduced at least 2-fold the expression of 263 and 98 genes, respectively. Not surprisingly, several genes that encode ion channels and macromolecule transporters were affected. Interestingly, treatment with ICI 182,780 markedly altered the expression of genes related to extracellular matrix organization. Matrix metalloproteinase 7 (Mmp7), osteopontin (Spp1), and neuronal pentraxin 1 (Nptx1) were among the most altered genes in this category. Upregulation of Mmp7 and Spp1 and downregulation of Nptx1 were validated by Northern blot. Increase in Mmp7 expression was further confirmed by immunohistochemistry and probably accounted for the decrease in collagen content observed in the efferent ductules of ICI 182,780-treated animals. Downregulation of Nptx1 probably contributed to the extracellular matrix changes and decreased amyloid deposition in the efferent ductules of ICI 182,780-treated animals. Identification of new molecular targets for estrogen action may help elucidate the regulatory role of this hormone in the male reproductive tract.
PMID:18495684 Yasuhara F et al; Biol Reprod 79 (3): 432-41 (2008)
Fulvestrant is a pure antiestrogen that emerged from a systematic medicinal chemistry strategy of modification of long-chain alkyl substitutes in the 7a-position of estradiol. Fulvestrant has no uterotrophic effects on the immature or ovariectomized rat and blocks the agonistic effects of estradiol and tamoxifen in a dose-dependent manner. In in vivo and in vitro breast cancer models, fulvestrant has anticancer activity at least as good as tamoxifen and is superior to tamoxifen in some models. Fulvestrant requires intramuscular administration in a proprietary formulation of castor oil and alcohols. When fulvestrant binds to estrogen receptor monomers it inhibits receptor dimerization, activating function 1 (AF1) and AF2 are rendered inactive, translocation of receptor to the nucleus is reduced, and degradation of the estrogen receptor is accelerated. This results in pure antiestrogenic effects. ...
PMID:15865849 Carlson RW; Clin Breast Cancer 6 Suppl 1: S5-8 (2005)
Estrogen and tamoxifen activate large conductance Ca(2+)-activated K(+) (BK(Ca)) channels in smooth muscle through a non-genomic mechanism that depends on the regulatory beta1 subunit and an extracellular binding site. It is unknown whether a "pure" anti-estrogen such as ICI 182,780 (Faslodex), that has no known estrogenic properties, would have any effect on BK(Ca) channels. Using single channel patch clamp techniques on canine colonic myocytes, the hypothesis that ICI 182,780 would activate BK(Ca) channels was tested. ICI 182,780 increased the open probability of BK(Ca) channels in inside-out patches with an EC(50) of 1 microM. These data suggest that molecules with the ability to bind nuclear estrogen receptors, regardless of oestrogenic or anti-estrogenic nature, activate BK(Ca) channels through this nongenomic, membrane-delimited mechanism. The identity and characteristics of this putative binding site remain unclear; however, it has pharmacological similarity to estrogen receptors alpha and beta, as ICI 182,780 interacts with it.
PMID:12145095 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1573435 Dick GM; Br J Pharmacol 136 (7): 961-4 (2002)
For more Mechanism of Action (Complete) data for FULVESTRANT (6 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
45
PharmaCompass offers a list of Fulvestrant API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fulvestrant manufacturer or Fulvestrant supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fulvestrant manufacturer or Fulvestrant supplier.
PharmaCompass also assists you with knowing the Fulvestrant API Price utilized in the formulation of products. Fulvestrant API Price is not always fixed or binding as the Fulvestrant Price is obtained through a variety of data sources. The Fulvestrant Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fulvestrant manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fulvestrant, including repackagers and relabelers. The FDA regulates Fulvestrant manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fulvestrant API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fulvestrant manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fulvestrant supplier is an individual or a company that provides Fulvestrant active pharmaceutical ingredient (API) or Fulvestrant finished formulations upon request. The Fulvestrant suppliers may include Fulvestrant API manufacturers, exporters, distributors and traders.
click here to find a list of Fulvestrant suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fulvestrant DMF (Drug Master File) is a document detailing the whole manufacturing process of Fulvestrant active pharmaceutical ingredient (API) in detail. Different forms of Fulvestrant DMFs exist exist since differing nations have different regulations, such as Fulvestrant USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fulvestrant DMF submitted to regulatory agencies in the US is known as a USDMF. Fulvestrant USDMF includes data on Fulvestrant's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fulvestrant USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fulvestrant suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Fulvestrant Drug Master File in Japan (Fulvestrant JDMF) empowers Fulvestrant API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Fulvestrant JDMF during the approval evaluation for pharmaceutical products. At the time of Fulvestrant JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Fulvestrant suppliers with JDMF on PharmaCompass.
A Fulvestrant CEP of the European Pharmacopoeia monograph is often referred to as a Fulvestrant Certificate of Suitability (COS). The purpose of a Fulvestrant CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Fulvestrant EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Fulvestrant to their clients by showing that a Fulvestrant CEP has been issued for it. The manufacturer submits a Fulvestrant CEP (COS) as part of the market authorization procedure, and it takes on the role of a Fulvestrant CEP holder for the record. Additionally, the data presented in the Fulvestrant CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Fulvestrant DMF.
A Fulvestrant CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Fulvestrant CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Fulvestrant suppliers with CEP (COS) on PharmaCompass.
A Fulvestrant written confirmation (Fulvestrant WC) is an official document issued by a regulatory agency to a Fulvestrant manufacturer, verifying that the manufacturing facility of a Fulvestrant active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Fulvestrant APIs or Fulvestrant finished pharmaceutical products to another nation, regulatory agencies frequently require a Fulvestrant WC (written confirmation) as part of the regulatory process.
click here to find a list of Fulvestrant suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Fulvestrant as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Fulvestrant API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Fulvestrant as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Fulvestrant and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Fulvestrant NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Fulvestrant suppliers with NDC on PharmaCompass.
Fulvestrant Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fulvestrant GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fulvestrant GMP manufacturer or Fulvestrant GMP API supplier for your needs.
A Fulvestrant CoA (Certificate of Analysis) is a formal document that attests to Fulvestrant's compliance with Fulvestrant specifications and serves as a tool for batch-level quality control.
Fulvestrant CoA mostly includes findings from lab analyses of a specific batch. For each Fulvestrant CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fulvestrant may be tested according to a variety of international standards, such as European Pharmacopoeia (Fulvestrant EP), Fulvestrant JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fulvestrant USP).
